Published online Jul 21, 2005. doi: 10.3748/wjg.v11.i27.4173
Revised: October 1, 2004
Accepted: October 5, 2004
Published online: July 21, 2005
AIM: To investigate the effect of tetramethylpyrazine (TMP), an active compound from Ligustium Wollichii Franchat, on electrolyte transport across the distal colon of rodents and the mechanism involved
METHODS: The short-circuit current (ISC) technique in conjunction with pharmacological agents and specific inhibitors were used in analyzing the electrolyte transport across the distal colon of rodents. The underlying cellular signaling mechanism was investigated by radioimmunoassay analysis (RIA) and a special mouse model of cystic fibrosis.
RESULTS: TMP stimulated a concentration-dependent rise in ISC, which was dependent on both Cl- and HCO3-, and inhibited by apical application of diphenylamine-2,2’-dicarboxylic acid (DPC) and glibenclamide, but resistant to 4,4’-diisothiocyanatostilbene-2,2’-disulfonic acid disodium salt hydrate (DIDS). Removal of Na+ from basolateral solution almost completely abolished the ISC response to TMP, but it was insensitive to apical Na+ replacement or apical Na+ channel blocker, amiloride. Pretreatment of colonic mucosa with BAPTA-AM, a membrane-permeable selective Ca2+ chelator, did not significantly alter the TMP-induced ISC. No additive effect of forskolin and 3-isobutyl-1-methylxanthine (IBMX) was observed on the TMP-induced ISC, but it was significantly reduced by a protein kinase A inhibitor, H89. RIA results showed that TMP (1 mmol/L) elicited a significant increase in cellular cAMP production, which was similar to that elicited by the adenylate cyclase activator, forskolin (10 µmol/L). The TMP-elicited ISC as well as forskolin- or IBMX-induced ISC were abolished in mice with homozygous mutation of the cystic fibrosis transmembrane conductance regulator (CFTR) presenting defective CFTR functions and secretions.
CONCLUSION: TMP may stimulate cAMP-dependent and CFTR-mediated Cl- and HCO3- secretion. This may have implications in the future development of alternative treatment for constipation.
- Citation: He Q, Zhu JX, Xing Y, Tsang LL, Yang N, Rowlands DK, Chung YW, Chan HC. Tetramethylpyrazine stimulates cystic fibrosis transmembrane conductance regulator-mediated anion secretion in distal colon of rodents. World J Gastroenterol 2005; 11(27): 4173-4179
- URL: https://www.wjgnet.com/1007-9327/full/v11/i27/4173.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i27.4173
Constipation presents a massive impact on health services, and the epidemiology investigation has demonstrated that the prevalence of constipation in many areas around the world is significantly high[1,2]. Studies have shown that constipation results primarily from changes in gastrointestinal motility[3]. In addition, alterations in regulating fluid and electrolyte transport across gut epithelia may represent another contributing factor in the pathophysiology of constipation[4].
The mammalian colon is the final station of the gastrointestinal tract, where the organism has the last opportunity to modify the electrolyte contents in the feces with balanced absorptive and secretory activities. The epithelial layer covering the inner surface of colon is a typical electrolyte-transporting epithelium, which under physiological conditions absorbs water, sodium and chloride while secreting NaCl as well as potassium and bicarbonate through a number of ion channels, carriers, and pumps, located either on the luminal or on the basolateral membrane. Epithelial Na+ channels populated in the apical membranes of the absorptive cells in the colon, which are amiloride-sensitive and well-known targets for mineralocorticoids, are responsible for Na+ absorption[5]. Cystic fibrosis transmembrane conductance regulator (CFTR), a cAMP-dependent Cl- channel, is predominantly expressed in colonic crypts[6,7] and known to mediate both Cl-[8,9] and HCO3- secretion[10,11]. Disturbance of ion transport, like cholera toxin and cystic fibrosis (CF) may result in diarrhea[12] or constipation[13] with severe pathological consequences[9].
The traditional therapeutic proposals, such as the employment of cathartic medications, stool softeners, lubricant grease, and enemas have some effects on constipation, but their side effects have restricted their use[14].
In our previous study, we have observed that Bak Foong Pill, consisting of more than 20 herbal ingredients, a well known Chinese remedy widely used for treating gynecological disorders, exerts a stimulatory effect on anion secretion by the human colonic epithelia cell line T84 primarily via the cAMP pathway with minor contribution from the Ca2+ pathway[15]. This result highlights the potential for exploring an active component of Bak Foong Pills for treatment of constipation or other obstructive bowel syndromes in patients.
Tetramethylpyrazine (TMP, C8H12N2, molecular weight 136.20), also known as ligustrazine, is the active compound extracted from one of the component herbs of Bak Foong Pill, Ligustium Wollichii. TMP is used in clinic to treat cardiovascular disorders because of its multitude of biological effects such as improving blood circulation and metabolism of cardiac muscle, increasing coronary flow, decreasing oxygen consumption, lowering blood pressure and inhibiting thrombosis[16-18]. TMP may act as an inhibitor of phosphodiesterase (PDE) to increase intracellular cAMP thereby exerting its antiplatelet and vasodilatation effects[19]. On the other hand, the effect of TMP on the release of calcium from internal stores has also been reported[17]. Therefore, it is possible that TMP may be the active compound responsible for mediating the effect of Bak Foong Pills. The focus of this study was to investigate the effect of TMP on water and electrolyte transport properties of native mammalian colon with the hope of exploring new alternative treatment for constipation or obstructive bowel syndromes.
TMP was purchased from the Fourth Pharmaceutical Factory of Beijing (Beijing, China). 3-Isobutyl-1-methylxanthine (IBMX), amiloride, DIDS, BAPTA-AM, POP and POPOP were obtained from Sigma Chemical Company (St. Louis, MO, USA). Calbiochem (San Diego, CA, USA) was the source for bumetanide, forskolin, TTX, H89, and glibenclamide. Diphenylamine-2,2’-dicarboxylic acid (DPC) was purchased from Riedel de Haen Chemicals (Hannover, Germany). cAMP measurement kit was purchased from the Institute of Atomic Energy Science (Beijing, China). Stock solutions of all the chemicals were dissolved in DMSO. Final DMSO concentrations never exceeded 1 mL/L. Preliminary experiments indicated that the vehicle did not alter any baseline electrophysiological parameters.
Krebs-Henseleit (KH) solution contained (in mmol/L): 117, NaCl; 4.7, KCl; 1.2, MgCl2; 1.2, KH2PO4; 24.8, NaHCO3; 2.5, CaCl2; 11.1, glucose. The solution was continuously bubbled with 95% O2 and 50 mL/L CO2 to maintain the pH at 7.4. In Cl- free solution, NaCl, KCl, and CaCl2 were replaced by sodium gluconate, potassium gluconate, and calcium gluconate, respectively[20]. When HCO3- free solution was used, NaHCO3 was replaced by NaCl, and the solution was buffered with 10 mmol/L Hepes with a pH of 7.4 when gassed with 100% O2. In the Cl- and HCO3- free solutions, NaCl and NaHCO3 were replaced by sodium gluconate, KCl by potassium gluconate, and CaCl2 by calcium gluconate. The solution was buffered with 10 mmol/L Hepes with a pH of 7.4 when gassed with 100% O2. In all solutions, the osmolarity was adjusted to 290 mOsm/L with D-mannitol if necessary.
Tissue preparation Adult male Sprague-Dawley rats (Laboratory Animal Services Center at The Chinese University of Hong Kong) ranging in age from 8 to 12 wk had free access to standard rodent laboratory food and water until the day of the experiments. The animals were killed by CO2 asphyxiation in accordance with a protocol approved by Animal Research Ethics Committee of the Chinese University of Hong Kong. Segments of distal colon about 5 cm proximal to the anus were quickly removed, cut along the mesenteric border into a flat sheet and flushed with ice-cold KH solution. The tissues were pinned flat with the mucosal side down in a Sylgard-lined petri dish containing ice-cold oxygenated solution. The serosa, submucosa, and muscular layer were stripped away with fine forceps to obtain a mucosa preparation. Two to three of these stripped mucosal preparations were obtained from one animal. TTX was used to exclude possible involvement of neuronal circuitry.
CFTR mice CFTR mice, B6.129P2-Cftr[tm1Unc/J], were purchased from Jackson’s Laboratory (Bar Harbor, ME 04609, USA) and then bred in Laboratory Animal Services Center at The Chinese University of Hong Kong by mating heterozygous pairs genotyped by PCR amplification of genomic DNA isolated from the animals’ tails, as described previously[21]. Homozygous CFTR mutation (-/-) mice and their littermate wild-type CFTR (+/+) controls were utilized in the study. Preparation of mouse colonic mucosa was similar to that described for the rat. Mice were cared for in accordance with the Laboratory Animal Services Center of the Chinese University of Hong Kong guidelines.
Short circuit current measurement The short-circuit current (ISC) was measured in vitro in Ussing chambers[22]. Flat sheets of distal colon with intact mucosa were mounted between two halves of modified Ussing chambers, in which the total cross-sectional area was 0.45 cm2. The mucosal and serosal surfaces of tissues were bathed with 10 mL of KH solution by recirculation from a reservoir maintained at 37°C during the experiment. The solution was gassed with 95% O2 and 50 mL/L CO2 to maintain the pH at 7.4. An equilibration period of 30 min was given before the experiments. The tissues were continuously short-circuited, and the transepithelial potential difference (PD) was measured through Ag/AgCl reference electrodes (World Precision Instruments, Sarasota, Boca Raton, FL, USA) connected to a preamplifier that was connected in turn to a voltage clamp amplifier (DVC 1000; World Precision Instruments). Voltage offset of two voltage electrodes and fluid resistance were adjusted and compensated prior to the onset of each measurement. At 0.5 intervals, a transepithelial PD of 0.1 mV (U) was applied to the tissue and the change in current (I) was measured. The tissue conductance (Gt) and the open-circuit PD could be calculated from these values according to Ohm’s law (Gt = I/U and PD = I/Gt). Drugs could be added directly to the apical or basolateral side of epithelium. Responses were continuously recorded on a chart recorder. The change in ISC was calculated on the basis of the value before and after stimulation and normalized as current per unit area of epithelium (µA/cm2), according to which, the total transmembrane charges (µC/cm2) for 30 min could be calculated. The methodology for tissue preparation and measurement of electrical parameters across the mucosal sheets was described earlier[23,24].
Measurement of cAMP Cytosolic cAMP concentrations were measured by radioimmunoassay analysis (RIA). After a 20-min period of equilibration in normal KH solution at 37°C, the isolated mucosal sheet was treated with TMP, forskolin, TMP with IBMX, TMP with IBMX and forskolin. The mucosal sheet without treatment could act as control. The mucosal sheets were further incubated for 2 min and then rapidly frozen in liquid nitrogen and weighed 50.0 mg frozen tissue precisely. The tissues were homogenized in 2 mL of 1 N perchloric acid using a glass homogenizer. The homogenate was centrifuged at 1 000 r/min, 600 μL of the supernatant was extracted and neutralized with 300 μL of 20% KOH. The sediment was discarded after centrifugation. Six hundred microliters of the supernatant was extracted and evaporated in an electrothermal blast drier at 80°C and the residue was dissolved in 200 μL TE buffer for further measurement. The amount of cAMP was determined by RIA with a 3H-labeled cAMP kit (Institute of Atomic Energy Science). Procedures were performed according to the manufacturer’s instructions.
Results were expressed as mean±SE. The number of experiments represented independent measurements on separate stripped mucosal preparation (two to three of these tissue preparations were obtained from one animal). Comparisons between groups of data were made by either the Student’s t-test (two-group comparison) or one-way ANOVA with Newman-Keuls post hoc test (three or more-group comparison). P < 0.05 was considered statistically significant. EC50 values were determined by nonlinear regression using GraphPad Prism software.
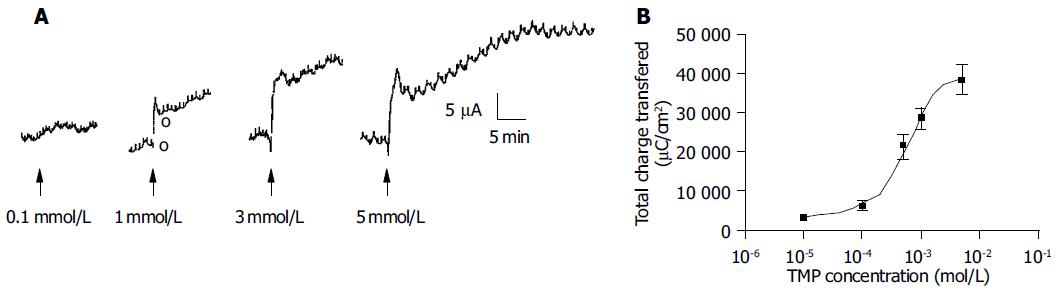
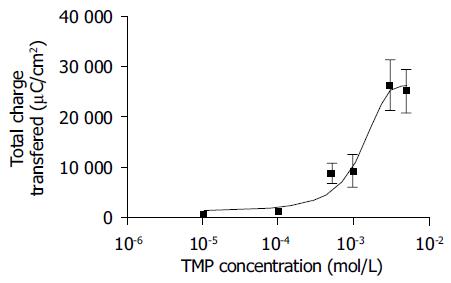
Addition of TMP to either the apical or basolateral membrane could elicit ISC responses with a greater response obtained with basolateral challenge. The present study only focused on the responses elicited by basolateral addition of TMP. As shown in Figure 1, the TMP response was concentration-dependent with an apparent EC50 of 0.994 mmol/L. At a concentration greater than 3 mmol/L, TMP induced a biphasic response with a fast transient peak followed by a sustained plateau.
To exclude possible involvement of enteric nervous system, 1 µmol/L TTX was added to the basolateral side before the administration of TMP and no significant change in the TMP-evoked increase in ISC was observed.
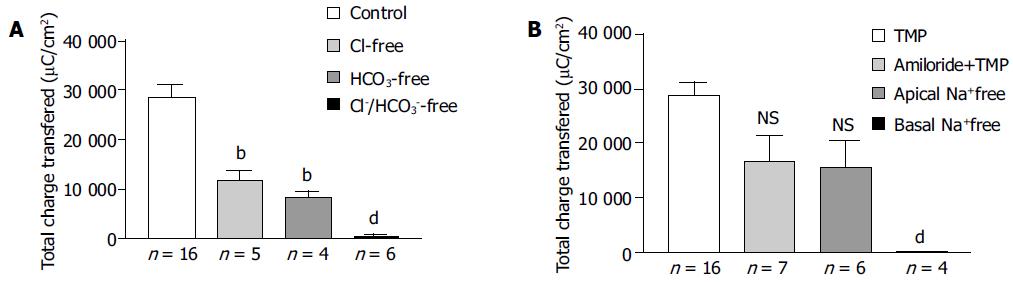
The increase in ISC could be attributed to either enhanced movement of positive charges from the luminal to the serosal side (i.e., Na+) or movement of negative charges into the lumen (i.e., Cl- and/or HCO3-). Ion substitution experiments were conducted to determine the ionic basis for the increases in ISC induced by TMP (Figure 2). When extracellular Cl- or HCO3- was removed, the TMP-induced response measured by total charge transfer per unit area over a 30-min period was reduced by 58.4% (n = 5, P < 0.01) and 70.8% (n = 4, P < 0.01), respectively. When both Cl- and HCO3- were replaced, 98.1% (n = 6, P < 0.001) of the response was abolished, indicating substantial contribution of Cl- and HCO3- to the TMP-induced response (Figure 2A).
To test whether the TMP-induced ISC responses were due to electrogenic Na+ absorption, apical Na+ was replaced but it did not reduce the TMP response (n = 6), excluding electrogenic Na+ absorption across the colonic mucosa. However, removing Na+ from basolateral or bilateral bathing solution abolished TMP-induced ISC response by 99.5% (n = 4, P < 0.001, Figure 2B), suggesting that both Cl- and HCO3- secretion might involve transport mechanisms that depend on basolateral Na+.
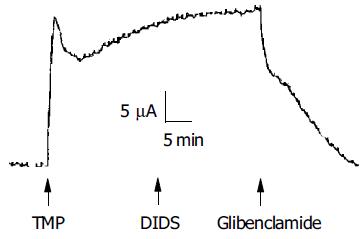
To investigate whether the TMP-induced ISC response was mediated by activation of the apical anion channel, different anion channel blockers were used. As shown in Figure 3, the TMP-induced ISC was completely inhibited by apical application of glibenclamide (1 mmol/L, n = 9), which has previously been shown to block CFTR[25] or DPC (2 mmol/L, n = 10, data not shown). However, the TMP-induced ISC response was not sensitive to apical addition of DIDS (100, 500 μmol/L, and 1 mmol/L, n = 5), known to block the Ca2+-activated Cl- channel.
Amiloride, an inhibitor of the epithelial Na+ channel, was also used to assess the involvement of Na+ absorption. In our preparation of rat colonic mucosa, a small amount of amiloride-sensitive current was observed under basal conditions. However, we did not detect any decrement of TMP-induced ISC response following apical amiloride (0.1 mmol/L) pretreatment (n = 7, Figure 2B).
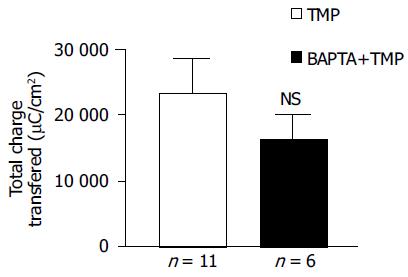
To address whether [Ca2+]i was involved in mediating TMP-evoked anion secretion, BAPTA-AM (100 μmol/L), a membrane-permeable selective Ca2+ chelator, was added bilaterally to the colonic mucosa for 45 min prior to the addition of TMP. The subsequent TMP-evoked ISC was slightly reduced but not significant (n = 6, P > 0.05, Figure 4).
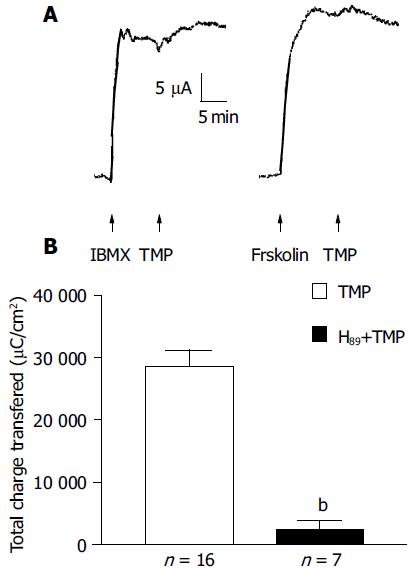
To test whether TMP exploited the cAMP pathway, the additive effect of forskolin (10 μmol/L, n = 7) or IBMX (100 μmol/L, n = 6) was examined. As shown in Figure 5A, no further increase in ISC by TMP was observed when the ISC was first elicited by the adenylate cyclase (AC) activator, forskolin, or PDE inhibitor, IBMX. Similarly, little or reduced forskolin or IBMX-induced ISC response was observed if the ISC was first activated by TMP (data not shown), indicating significant overlapping of the TMP signaling pathway with the cAMP-dependent one. This possibility was further tested by examining the effect of the protein kinase A (PKA) inhibitor, H89. The ISC response to TMP could be blocked by 91.7% upon bilateral pretreatment with 5 μmol/L H89 (n = 7, Figure 5B).
It is also possible that TMP may alter arachidonic acid metabolism affecting production of prostaglandins (PG), which are also known to be a mediator of other secretagogues of intestinal secretion[26]. Therefore, the effect of PG synthesis inhibitor, indomethacin, on TMP-induced ISC increase was also determined. In the presence of basolateral indomethacin (10 μmol/L), the TMP-induced ISC response reduced but substantial response remained with concentration-dependent characteristics (Figure 6). The indomethacin-insensitive TMP response could be inhibited by H89 (data not shown), consistent with the involvement of the cAMP pathway.
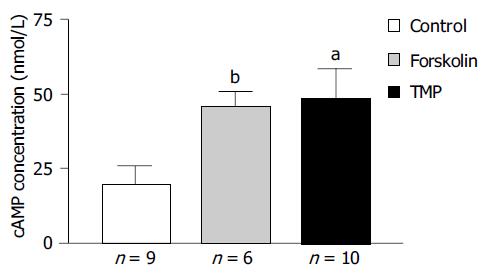
RIA was performed to study the effect of TMP on colonic epithelial intracellular cAMP levels and the results are shown in Figure 7. The intracellular cAMP content could be stimulated from a basal level of 20.0 ± 5.8 nmol/L (n = 9) to 45.8 ± 5.0 nmol/L (n = 6, P < 0.005) in response to AC activator forskolin (10 µmol/L), or to 48.8 ± 9.9 nmol/L (n = 10, P < 0.05) in response to TMP (1 mmol/L).
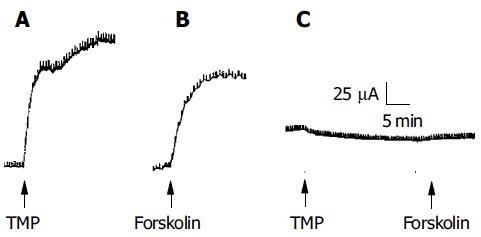
Since CFTR is a cAMP-dependent Cl- channel, the involvement of the cAMP-dependent pathway as shown above suggests that the secretory response elicited by TMP may be mediated by CFTR. To test this hypothesis, mutant CF mice with homozygous mutation of CFTR (-/-) were used and the effect of TMP on colonic mucosa was compared to that obtained from the wild type, CFTR (+/+). TMP (5 mmol/L) added to the basolateral side of the wild type colonic mucosa produced a sustained increase in ISC response (n = 3, Figure 8A), which mimicked the forskolin-induced ISC response (n = 3, Figure 8B). The same concentration of TMP was used to challenge colonic mucosa from CF mice and no TMP response was observed (n = 5, Figure 8C), neither with addition of forskolin (10 μmol/L, n = 3) or IBMX (100 μmol/L, n = 3), indicating that both TMP-induced Cl- and HCO3- secretion was mediated by CFTR.
The present study has demonstrated a stimulatory effect of TMP on colonic anion secretion but not Na+ absorption. The TMP-induced increase in ISC is not considered to be mediated by electrogenic Na+ absorption because it was not inhibited by apical addition of amiloride, the epithelial sodium channel blocker, or by Na+ removal from apical side. Previous studies have also shown that isolated rat distal colon is largely devoid of electrogenic Na+ transport[27]. However, replacement of extracellular Cl- and HCO3- ions, or addition of Cl- channel blockers, greatly attenuated the TMP-induced ISC, suggesting that TMP has a primary effect on the anion secretion by the colon of rodents. It is interesting to note that both Cl- and HCO3- secretions seem to be Na+-dependent since Na+ removal from basolateral solution almost completely abolished the TMP-induced ISC, suggesting involvement of basolateral Na+-dependent transporting mechanisms, i.e., both Na+-HCO3- cotransporter[28] and Na+-K+-2Cl- cotransporter[29] are implicated in secondary active Cl- and HCO3- secretion in many epithelia including the intestine.
The results of the present study suggest that CFTR is the likely candidate pathway for apical Cl- and HCO3- exit since the TMP-evoked anion secretion was completely inhibited by glibenclamide and DPC, which are known to block CFTR[25,30], but not by DIDS which has no effect on the activity and conductance of CFTR[31]. The strong evidence indicating the involvement of CFTR came from the studies on CF mice with CFTR homozygous mutation. The complete abolition of the TMP-induced response in CFTR mutated colonic mucosa suggests that both TMP-stimulated Cl- and HCO3- secretions are mediated by CFTR, which is consistent with an important role of CFTR in HCO3- as well as Cl- secretion in many epithelial tissues[32]. It is interesting to note that removing Cl- or HCO3- alone from the bathing solution resulted in 58.4% and 70.8% attenuation in TMP-induced ISC, respectively. The summated inhibition is more than 100%, suggesting interaction between the two ions, possibly during their transport by CFTR. The phenomena that the presence of one ion interferes with the transport of another ion have been previously reported to be associated with CFTR[33]. In native guinea pig pancreatic duct cells, CFTR is between 3-5 times more selective for Cl- over HCO3-; however, extracellular HCO3- significantly inhibits CFTR current carried by Cl-. The interaction between Cl- and HCO3- secretion may also have physiological bearing in the colon, the details of which, especially the role of CFTR, remain to be elucidated.
The involvement of CFTR also suggests that the effect of TMP is mediated primarily by the cAMP-dependent pathway since CFTR is a cAMP-activated Cl- channel[34] and mainly responsible for cAMP-dependent anion secretion[35]. The amount of cAMP available to activate PKA to phosphorylate CFTR is determined by the balance between cAMP production by AC and cAMP hydrolysis by PDE[36]. cAMP can be stimulated either by activation of AC or inhibition of PDE. The observation that TMP failed to further increase the ISC, or lack of additive effect after a maximal stimulation with forskolin (AC activator) or IBMX (PDE inhibitor) suggests that the signaling pathway mediating the effect of TMP may overlap with the cAMP pathway. These indicate that TMP, forskolin and IBMX share a common intracellular pathway (i.e., a rise in [cAMP]i). This notion is further supported by the inhibition of the TMP responses by H89, an inhibitor of PKA, which is the primary target for intracellular cAMP. The present results obtained from RIA studies demonstrate an enhancement of intracellular cAMP by TMP in the rat colon.
While there is no question about the involvement of cAMP in mediating the TMP effect, how TMP activates intracellular cAMP remains to be elucidated. Previous studies reported that the antiplatelet activity of TMP is due to the enhancement of intracellular cAMP by acting as a PDE inhibitor[19]. Although we do not have any substantial evidence to support this, the asymmetrical ISC response to apical and basolateral challenge of TMP suggests differential distribution of the component(s) of the signaling pathway involved. In this regard, it is interesting to note that PDE subtypes are differently localized in the apical and basolateral membrane domains of T84 colonic epithelial cells[8]. Therefore, the more potent effect of TMP observed when applied to the basolateral membrane can be explained by the presence of greater amount of a certain PDE subtype in the basolateral domain of the colonic mucosa, if TMP is indeed acting as a PDE inhibitor. Further studies along this line are necessary in order to understand the detail mechanism of cAMP activation by TMP.
It should be mentioned that while the TMP effect does not appear to involve the enteric nervous system, portion of the TMP effect could be blocked by indomethacin, an inhibitor of PG synthesis, indicating the involvement of PG which is well known for its function as the mediator of other secretagogues and acts by stimulating AC in the intestinal epithelium[37]. The present studies also demonstrate that both the indomethacin-sensitive and indomethacin-insensitive TMP response could be blocked by H89 indicating the involvement of cAMP in both cases. Therefore, TMP may indirectly activate cAMP via PG, apart from its possible direct action as a PDE inhibitor.
Although previous studies demonstrating effects of TMP on cardiac vascular function[17] have suggested its action on the Ca2+ pathway, the present results suggest that TMP acts primarily on the cAMP but not on the Ca2+ pathway, at least for the concentration range used in the present study. However, it should be noted that the treatment with BAPTA-AM in the present study did appear to reduce the TMP response although the effect was not significant. Therefore, TMP might have a minor action on the Ca2+-dependent pathway, which may also be TMP concentration dependent. We have preliminary data indicating increased Ca2+ involvement with increased TMP concentrations. Further studies using direct Ca2+ measurement may be required to clarify this.
The effect of TMP on colonic anion secretion by a primary action on the cAMP pathway is consistent with our previous studies with Bak Foong Pills, indicating that TMP may be an active compound responsible for mediating the stimulatory effect of Bak Foong Pills on colonic anion secretion previously observed[15]. Since Cl- secretion is the main driving force for fluid secretion across the colon[9], and HCO3- secretion at the luminal surface is considered to have a decisive influence on a constant pH microclimate sustenance[38], the ability of TMP to enhance the secretion of these anions secretion may have implications in future development of alternative treatment for constipation. Most importantly, as an active compound of many popular traditional Chinese medicines, TMP has been in clinical use with trivial side effects and few clinically relevant drug interactions reported. Taken together, TMP may have therapeutic potential for treatment of constipation caused by disturbance of electrolyte and fluid transport in the GI tract.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Pan G, Lu S, Ke M, Han S, Guo H, Fang X. Epidemiologic study of the irritable bowel syndrome in Beijing: stratified randomized study by cluster sampling. Chin Med J (Engl). 2000;113:35-39. [PubMed] |
| 2. | Bommelaer G, Dorval E, Denis P, Czernichow P, Frexinos J, Pelc A, Slama A, El Hasnaoui A. Prevalence of irritable bowel syndrome in the French population according to the Rome I criteria. Gastroenterol Clin Biol. 2002;26:1118-1123. [PubMed] |
| 3. | Belai A, Wheeler H, Burnstock G. Innervation of the rat gastrointestinal sphincters: changes during development and aging. Int J Dev Neurosci. 1995;13:81-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Burleigh DE. Evidence for a functional cholinergic deficit in human colonic tissue resected for constipation. J Pharm Pharmacol. 1988;40:55-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Dawson DC. Ion channels and colonic salt transport. Annu Rev Physiol. 1991;53:321-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Sood R, Bear C, Auerbach W, Reyes E, Jensen T, Kartner N, Riordan JR, Buchwald M. Regulation of CFTR expression and function during differentiation of intestinal epithelial cells. EMBO J. 1992;11:2487-2494. [PubMed] |
| 7. | Strong TV, Boehm K, Collins FS. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J Clin Invest. 1994;93:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 202] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | O'Grady SM, Jiang X, Maniak PJ, Birmachu W, Scribner LR, Bulbulian B, Gullikson GW. Cyclic AMP-dependent Cl secretion is regulated by multiple phosphodiesterase subtypes in human colonic epithelial cells. J Membr Biol. 2002;185:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245-289. [PubMed] |
| 10. | Clarke LL, Harline MC. Dual role of CFTR in cAMP-stimulated HCO3- secretion across murine duodenum. Am J Physiol. 1998;274:G718-G726. [PubMed] |
| 11. | Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossmann H, Colledge WH, Evans M, Ratcliff R, Gregor M. A functional CFTR protein is required for mouse intestinal cAMP-, cGMP- and Ca(2+)-dependent HCO3- secretion. J Physiol. 1997;505:411-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 201] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Lencer WI, Delp C, Neutra MR, Madara JL. Mechanism of cholera toxin action on a polarized human intestinal epithelial cell line: role of vesicular traffic. J Cell Biol. 1992;117:1197-1209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Ewe K. Intestinal transport in constipation and diarrhoea. Pharmacology. 1988;36 Suppl 1:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Campillos Páez MT, Vallés Ugarte ML, San Laureano Palomero T, Pérez Hernánsaiz M. [Constipation and laxative consumption in the elderly]. Aten Primaria. 2000;26:430-432. [PubMed] |
| 15. | Zhu JX, Chan YM, Tsang LL, Chan LN, Zhou Q, Zhou CX, Chan HC. Cellular signaling mechanisms underlying pharmacological action of Bak Foong Pills on gastrointestinal secretion. Jpn J Physiol. 2002;52:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Xu J, Li YK, Liang ZJ. Effects of tetramethylpyrazine and ferulic acid alone or combined on vascular smooth muscle, blood viscosity and toxicity. Zhongguo Zhong Yao Za Zhi. 1992;17:680-62, 680-62. [PubMed] |
| 17. | Liu SY, Sylvester DM. Antiplatelet activity of tetramethylpyrazine. Thromb Res. 1994;75:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Lin LN, Wang WT, Xu ZJ. Clinical study on ligustrazine in treating myocardial ischemia and reperfusion injury. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1997;17:261-263. [PubMed] |
| 19. | Lin CI, Wu SL, Tao PL, Chen HM, Wei J. The role of cyclic AMP and phosphodiesterase activity in the mechanism of action of tetramethylpyrazine on human and dog cardiac and dog coronary arterial tissues. J Pharm Pharmacol. 1993;45:963-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Kenyon JL, Gibbons WR. Effects of low-chloride solutions on action potentials of sheep cardiac Purkinje fibers. J Gen Physiol. 1977;70:635-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 131] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 697] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 22. | Ussing HH, Zerahn K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951;23:110-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1721] [Cited by in RCA: 1603] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 23. | Strabel D, Diener M. Evidence against direct activation of chloride secretion by carbachol in the rat distal colon. Eur J Pharmacol. 1995;274:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Andres H, Rock R, Bridges RJ, Rummel W, Schreiner J. Submucosal plexus and electrolyte transport across rat colonic mucosa. J Physiol. 1985;364:301-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Schultz BD, DeRoos AD, Venglarik CJ, Singh AK, Frizzell RA, Bridges RJ. Glibenclamide blockade of CFTR chloride channels. Am J Physiol. 1996;271:L192-L200. [PubMed] |
| 26. | Mall M, Bleich M, Schürlein M, Kühr J, Seydewitz HH, Brandis M, Greger R, Kunzelmann K. Cholinergic ion secretion in human colon requires coactivation by cAMP. Am J Physiol. 1998;275:G1274-G1281. [PubMed] |
| 27. | Foster ES, Zimmerman TW, Hayslett JP, Binder HJ. Corticosteroid alteration of active electrolyte transport in rat distal colon. Am J Physiol. 1983;245:G668-G675. [PubMed] |
| 28. | Bachmann O, Rossmann H, Berger UV, Colledge WH, Ratcliff R, Evans MJ, Gregor M, Seidler U. cAMP-mediated regulation of murine intestinal/pancreatic Na+/HCO3- cotransporter subtype pNBC1. Am J Physiol Gastrointest Liver Physiol. 2003;284:G37-G45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Haas M, Forbush B. The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol. 2000;62:515-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 304] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 30. | Ito Y, Mizuno Y, Aoyama M, Kume H, Yamaki K. CFTR-Mediated anion conductance regulates Na(+)-K(+)-pump activity in Calu-3 human airway cells. Biochem Biophys Res Commun. 2000;274:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Greger R. Role of CFTR in the colon. Annu Rev Physiol. 2000;62:467-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Geibel JP, Singh S, Rajendran VM, Binder HJ. HCO(3)(-) secretion in the rat colonic crypt is closely linked to Cl(-) secretion. Gastroenterology. 2000;118:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Gray MA, O'Reilly C, Winpenny J, Argent B. Functional interactions of HCO3- with cystic fibrosis transmembrane conductance regulator. JOP. 2001;2:207-211. [PubMed] |
| 34. | Dalemans W, Barbry P, Champigny G, Jallat S, Dott K, Dreyer D, Crystal RG, Pavirani A, Lecocq JP, Lazdunski M. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature. 1991;354:526-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 560] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 35. | Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 379] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 36. | Kopito RR. Biosynthesis and degradation of CFTR. Physiol Rev. 1999;79:S167-S173. [PubMed] |
| 37. | Craven PA, DeRubertis FR. Stimulation of rat colonic mucosal prostaglandin synthesis by calcium and carbamylcholine: relationship to alterations in cyclic nucleotide metabolism. Prostaglandins. 1981;21:65-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Genz AK, v Engelhardt W, Busche R. Maintenance and regulation of the pH microclimate at the luminal surface of the distal colon of guinea-pig. J Physiol. 1999;517:507-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |