Published online Jul 21, 2005. doi: 10.3748/wjg.v11.i27.4161
Revised: January 1, 2004
Accepted: January 5, 2004
Published online: July 21, 2005
AIM: To investigate the ability of a genetically altered embryonic stem (ES) cell line to generate insulin-producing cells in vitro following transfer of the Nkx2.2 gene.
METHODS: Hamster Nkx2.2 genes were transferred into mouse ES cells. Parental and Nkx2.2-transfected ES cells were initiated toward differentiation in embryoid body (EB) culture for 5 d and the resulting EBs were transferred to an attached culture system. Dithizone (DTZ), a zinc-chelating agent known to selectively stain pancreatic beta cells, was used to detect insulin-producing cells. The outgrowths were incubated in DTZ solution (final concentration, 100 μg/mL) for 15 min before being examined microscopically. Gene expression of the endocrine pancreatic markers was also analyzed by RT-PCR. In addition, insulin production was determined immunohistochemically and its secretion was examined using an ELISA.
RESULTS: DTZ-stained cellular clusters appeared after approximately 14 d in the culture of Nkx2.2-transfected ES cells (Nkx-ES cells), which was as much as 2 wk earlier, than those in the culture of parental ES cells (wt-ES). The frequency of DTZ-positive cells among total cultured cells on day 28 accounted for approximately 1.0% and 0.1% of the Nkx-ES- and wt-ES-derived EB outgrowths, respectively. The DTZ-positive cellular clusters were found to be immunoreactive to insulin, while the gene expressions of pancreatic-duodenal homeobox 1 (PDX1), proinsulin 1 and proinsulin 2 were observed in the cultures that contained DTZ-positive cellular clusters. Insulin secretion was also confirmed by ELISA, whereas glucose-dependent secretion was not demonstrated.
CONCLUSION: Nkx2.2-transfected ES cells showed an ability to differentiate into insulin-producing cells.
- Citation: Shiroi A, Ueda S, Ouji Y, Saito K, Moriya K, Sugie Y, Fukui H, Ishizaka S, Yoshikawa M. Differentiation of embryonic stem cells into insulin-producing cells promoted by Nkx2.2 gene transfer. World J Gastroenterol 2005; 11(27): 4161-4166
- URL: https://www.wjgnet.com/1007-9327/full/v11/i27/4161.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i27.4161
It has been proposed that approximately 150 million people worldwide have diabetes mellitus, which may double by 2025, and 5-10% of those suffer from type 1 diabetes, for which injections of insulin are unavoidable. Transplantation of pancreatic islets for type 1 diabetes is a promising therapeutic strategy[1], however, an inadequate supply of donor islets is a major obstacle. Thus, embryonic stem (ES) cells are being considered as a potential source for generating insulin-producing cells. ES cells come from clonal cell lines derived from the inner cell mass of developing blastocysts[2,3], and are able to proliferate in vitro and have shown a capacity to differentiate into a broad spectrum of derivatives of all three embryonic germ layers including hematopoietic cells, cardiomyocytes, smooth muscle cells, neurons, hepatocytes, and insulin-producing cells . We recently reported the appearance of the islet-like cellular clusters containing insulin-producing cells in embryoid body (EB) outgrowth cultures with the use of a zinc-chelating substance, dithizone (DTZ)[11]. However, the development of islet-like cellular clusters was found in only a small portion of the EB outgrowths and required long-term cultures of more than 3 wk.
Nkx2.2 and Nkx6.1 are NK-homeodomain genes expressed in early pancreatic progenitor cells as well as neurogenin-3 (Ngn3)-expressing islet precursor cells, and are considered to be β-cell competence factors[12-14]. Nkx2.2 mutants completely lack insulin expression[14], while Nkx6.1 mutants show a less dramatic impaired differentiation of β-cells[15]. Further, Nkx2.2 expression lasts for a longer period of time in differentiated islet cells. These findings suggest that Nkx2.2 is a critical transcription factor in early pancreatic endocrine development and the following differentiation into pancreatic β-cells.
In the present study, we generated Nkx2.2-expressing cell lines by transfection of the Nkx2.2 gene into undiffer-entiated ES cells, and investigated their ability to differentiate into insulin-producing cells in EB outgrowth cultures. We found that insulin-producing cells appeared as much as 2 wk earlier in the EB outgrowths derived from Nkx2.2-transfected ES (Nkx-ES) cells than in those from parental ES (wt-ES) cells. Further, the frequency of insulin-producing cells on culture d 28 was approximately 1% in the Nkx-ES-derived EB outgrowths, a 10-fold greater efficiency as compared to that on the wt-ES-derived EB outgrowths. These results suggest that Nkx2.2 acts to promote the differentiation of ES cells into insulin-producing cells.
We utilized a mouse ES cell line, EB3 (129/SvJ mouse ES cells, a kind gift from Dr. Hitoshi Niwa, RIKEN Center for Developmental Biology, Kobe, Japan)[15], which was a subline derived from E14tg2a ES cells[16] and carried the blasticidin S-resistant selection marker gene driven by the Oct-3/4 promoter (active under undifferentiated status)[17]. Undifferentiated EB3 cells were maintained on gelatin-coated dishes without feeder cells in the maintenance medium, which was knockout-DMEM medium (Gibco-BRL) supplemented with 10% fetal bovine serum (FBS; GIBCO/BRL), 0.1 mmol/L of 2-mercaptoethanol (Sigma), 10 mmol/L of non-essential amino acids (GIBCO/BRL), L-glutamine, and 1 000 U/mL of leukemia inhibitory factor (LIF; GIBCO/BRL).
The hamster Nkx2.2 gene was obtained from the plasmid pBAT12.shNkx2.2 (kindly provided by Dr. M. German, University of California, San Francisco). The cDNA was inserted into the BstXI-stuffer site of the expression vector pPyCAGIRESzeocinpA[18]. The constructed plasmid pPyCAGIRESzeocinpA-Nkx2.2 contained the zeocin resistance gene driven by the chicken β-actin promoter. The Nkx2.2 vector (20 µg) was transfected using a lipofection method with lipofectAmine (GIBCO/BRL). Transfected ES cells were selected by growth in the presence of 20 µg/mL of zeocin, and three clones expressing Nkx2.2 (Nkx-ES) were established. Undifferentiated Nkx-ES cells were maintained in the same manner as undifferentiated wt-ES cells.
The method used for the EB outgrowths has been previously described[11]. Briefly, undifferentiated ES cells were dissociated into single-cell suspensions and then cultured in hanging drops to induce embryoid body (EB) formation at an initial cell density of 500 cells per drop (20 µL) of ES cell-medium in the absence of LIF. After 5 d in a hanging drop culture, the resulting EBs were plated in plastic 30-mm gelatin-coated dishes (five EBs per dish), and then allowed to attach and form outgrowth cultures. A half amount of culture medium was replenished with new medium every 2 d. The culture medium used was the maintenance medium lacking LIF and known differentiation-inducing factors. Throughout the whole culture, no growth factors or cytokines including insulin were added to the culture medium.
A DTZ (Merck, Whitehouse Station, NJ, USA) stock solution was prepared with 50 mg of DTZ in 5 mL of dimethylsulfoxide (DMSO) and stored briefly at -15°C. The staining solution was filtered through a 0.2 µm nylon filter and then used as a DTZ working solution. In vitro DTZ staining was performed by adding 10 µL of the stock solution to 1 mL of culture medium, then the culture dishes were incubated at 37°C for 15 min in the DTZ solution. After the dishes were rinsed thrice with HBSS, clusters stained crimson red were examined using a stereomicroscope. After examination, the dishes were refilled with DMEM containing 10% FBS. Although the stain completely disappeared from the cells after 5 h, the cultures that had not been treated with DTZ were used for RT-PCR and insulin secretion studies to avoid the influence of the treatment. In some experiments, the number of DTZ-stained cells in the cultures was determined by counting crimson red cells after trypsinization following DTZ stain.
Total RNA was extracted from the cells using TRIzol (GIBCO/BRL). DNase-treated total RNA was used for the first-strand cDNA. This reaction was performed using M-MLV Reverse Transcriptase (Promega) and Random Primer (Takara Bio Inc.), following the protocols of the manufacturers. cDNA samples were subjected to PCR amplification with specific primers under linear conditions in order to reflect the original amount of the specific transcript. The cycling parameters were as follows: denaturation at 94°C for 1 min, annealing at 52-60°C for 1 min (depending on the primer), elongation at 72°C for 1 min (35 cycles), and a final extension for 20 min at 72°C. The PCR primers and the lengths of the amplified products were as follows: β-actin (TGAACTGGCTGACTGCTGTG and CATCCTTGGCCTCAGCATAG, 174 bp); pro-insulin 1 (GTTGGTGCACTTCCTACCCCTG and GTAGAG-GGAGCAGATGCTGGTG, 300 bp); pro-insulin 2 (GT-GGATGCGCTTCCTGCCCCTG and GTAGAGGG-AGCAGATGCTGGTG, 300 bp); PDX1 (GGCCACACA-GCTCTACAAGG and TTCCACTTCATGCGACGGTT, 582 bp); and mouse-nkx2.2 (TGACCAACACAAAG-ACGGGGT and GCACGTTTCATCTTGTAGCGA, 650 bp). A set of mouse-nkx2.2 primers was used for the detection of hamster Nkx2.2.
Cells in culture dishes were fixed with 4% paraformaldehyde in phosphate-buffered solution and immunocytochemistry was carried out using a standard protocol. As the primary antibody for Nkx2.2, mouse anti-chick Nkx2.2 mAb (Hybridoma Bank, University of Iowa) was used at a dilution of 1:40. For detection of the primary antibody, a biotinylated anti-mouse antibody, ABC kit (VECTASTAIN Elite ABC KIT), and DAB (Dojindo; Kumamoto, Japan) were utilized according to the manufacturers’ instructions.
For detection of the primary antibody, a fluorescent labeled secondary antibody, goat anti-guinea pig IgG conjugated with fluorescein-5-isothiocyanate (Cappel 57000, ICN Pharmaceuticals, Inc., OH, USA), was utilized according to the manufacturer’s instructions. All nuclei were stained with DAPI (Dojindo; Kumamoto, Japan).
Cells in the culture dishes were washed thrice with PBS and pre-incubated in Krebs Ringer bicarbonate buffer (KRBB) containing 2.8 mmol/L of glucose for 1 h, then placed in 1 000 μL of KRBB with 2.8 mmol/L of glucose for 2 h. Next, the supernatant was collected and the dishes were rinsed thrice with PBS, after which the cells were re-incubated with KRBB containing 25.5 mmol/L of glucose for 2 h. Subsequently, conditioned medium samples were collected, and insulin levels were measured using an enzyme immunoassay (Mouse Insulin ELISA TMB Kit AKRIN-011T, Shibayagi Co., Ltd. Gunma, Japan) that detects mouse insulin in a range between 156 and 10 000 pg/mL with no cross-reactivity to C-peptide.
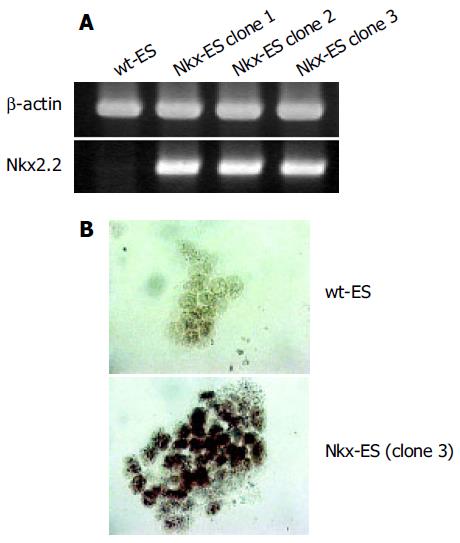
ES cells were transfected with the Nkx2.2 expression vector and selected with zeocin. Three stable clones at a time were obtained and screened for Nkx2.2 expression by RT-PCR and three clones with a high expression of Nkx2.2 were finally selected (Figure 1A). Subsequently, immunocytochemistry showed that all three clones expressed Nkx2.2 protein (Figure 1B). Each Nkx-ES clone demonstrated similar results in the following experiments, thus representative results from only clone 3 are shown.
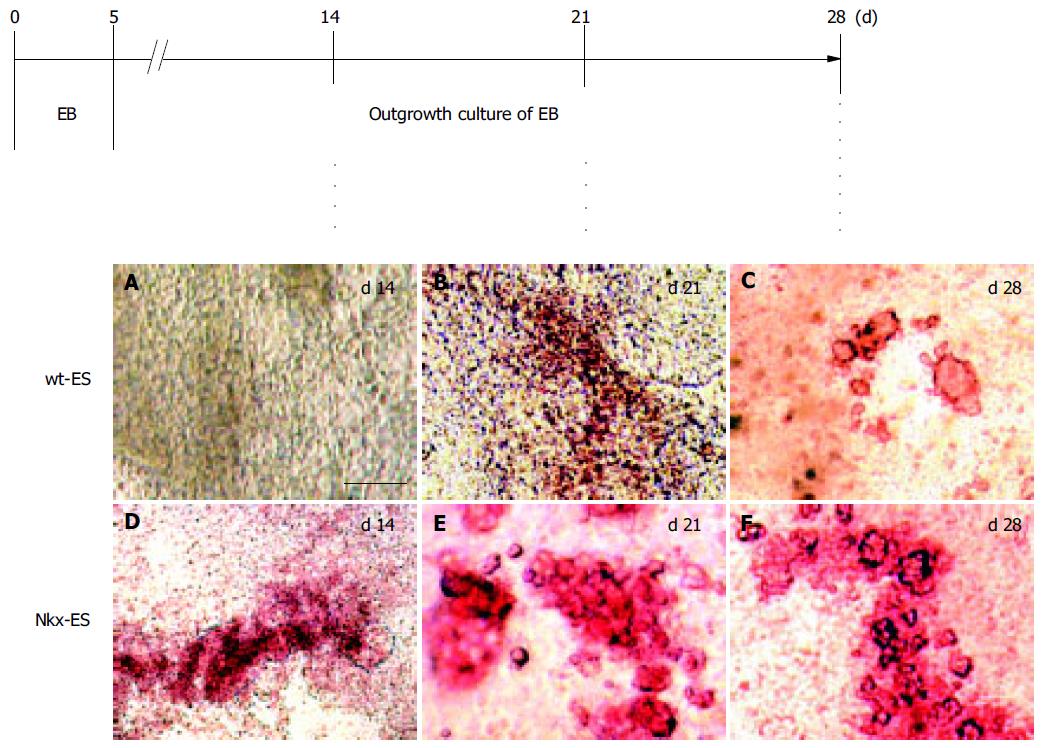
DTZ, a zinc-chelating agent, is known to selectively stain pancreatic β-cells crimson red[19-21] and we previously demonstrated that DTZ stain could also be applied for the detection of ES-derived insulin-producing cells. Figure 2 shows the outline of the EB outgrowth culture and results of DTZ-staining. DTZ-positive cellular clusters appeared as much as 2 wk earlier in the EB outgrowths derived from Nkx-ES cells. On d 14, DTZ-positive cells were already present in the EB outgrowths derived from Nkx-ES cells (Figure 2D), whereas they were absent in those from wt-ES cells (Figure 2A). On d 21, distinct DTZ-positive cellular clusters were observed in the Nkx-ES-derived EB outgrowths (Figure 2E), while they were obscure in the differentiating wt-ES cultures (Figure 2B). In the wt-ES-derived EB outgrowths, DTZ-positive cellular clusters became distinct on d 28 (Figure 2C). The appearance of DTZ-positive clusters on d 28 in Nkx-ES-derived EB outgrowths was quite similar to that seen on d 21 (Figures 2E and F).
DTZ-stained clusters that appeared in Nkx-ES-derived EB outgrowths were frequently larger than those in wt-ES-derived EB outgrowths. To estimate the frequency of the emerged DTZ-stained cells in the cultures, the number of DTZ-stained red cells was directly counted under a microscope after trypsinization following the treatment with DTZ. The percentages of DTZ-stained cells among total cells on day 28 were 1.0 ± 0.2% of the Nkx-ES-derivatives and less than 0.1% of the wt-ES-derivatives.
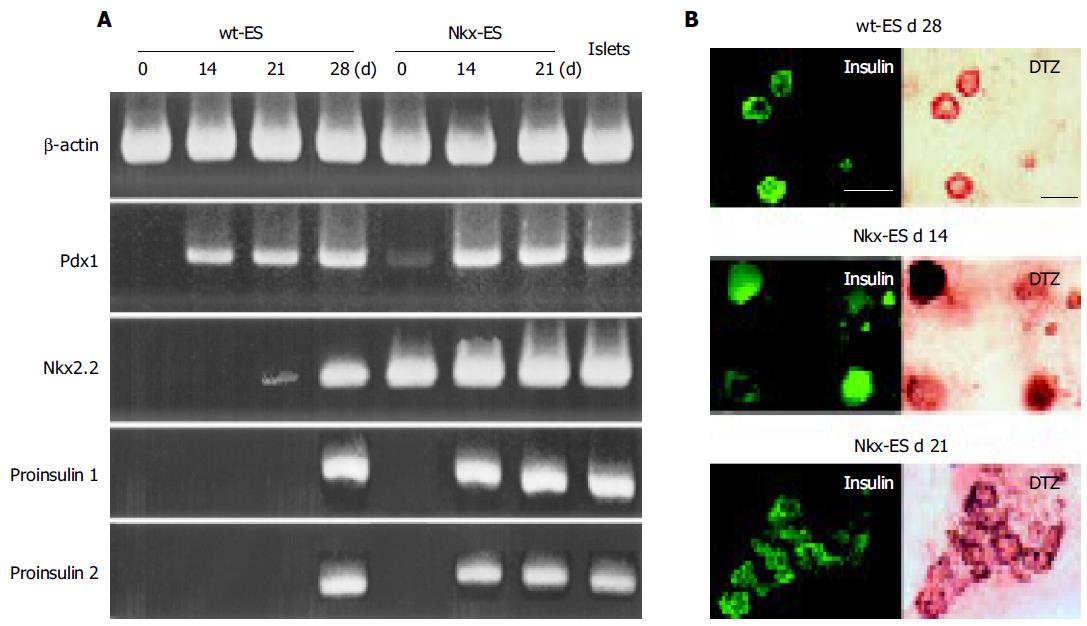
Pdx 1, a key transcriptional factor of pancreatic differentiation, was faintly detected in Nkx-ES cells and completely absent in wt-ES cells on d 0. Thereafter, Pdx 1 was expressed in all EB outgrowths on d 14, 21, and 28 as well as in isolated mouse islets. In contrast, Nkx2.2 was not detected on d 14, faintly detected on d 21, and clearly detected on d 28 in the differentiating wt-ES cells. In addition, pro-insulin 1 and 2 were detected on d 14 in the Nkx-ES-derived EB outgrowths, though not until d 28 in the wt-ES-derived EB outgrowths. To confirm the expression of insulin protein, we performed an immunohistochemistry examination in parallel with the DTZ-stain examination (Figure 3B). Immunoreactivity against insulin was found to coincide with the DTZ-stained culture areas within the EB outgrowths. In Nkx-ES-derived EB outgrowths, insulin-immunoreactivity was detected as early as on d 14, whereas it did not become positive until d 28 in the wt-ES-derived EB outgrowths. No immunoreactivity was found on d 14 or 21 in the wt-ES-derived EB outgrowths.
EB outgrowths on d 28 were used for the detection of released insulin. Insulin concentrations in culture media of wt-ES-derived EB outgrowths incubated with 2.8 and 25 mmol/L of glucose were 163 ± 38 and 191 ± 31 pg/mL, respectively, while those in media of Nkx-ES-derived EB outgrowths were 525 ± 132 and 660 ± 171 pg/mL with 25.5 mmol/L of glucose, respectively. Glucose-dependent insulin secretion was not observed in wt-ES- or Nkx-ES- derived EB outgrowth cultures, whereas secreted insulin was detected in the Nkx-ES-derived EB outgrowth cultures, in amounts several times greater than in the wt-ES-derived EB outgrowth cultures.
The homeodomain transcription factor Nkx2.2 is one of the key transcription factors in pancreatic β -cell differentiation and expressed throughout all development periods, as well as broadly in the initial pancreatic precursor population, and in Ngn3-expressing islet progenitor cells and differentiated islet cells[12,13,22]. Mice homozygous for a null mutation of Nkx2.2 develop severe hyperglycemia leading to death shortly after birth, because of the lack of insulin-producing β-cells[14], and show a variety of functional defects in the islet cells that produce endocrine hormones other than insulin. In the present study, we examined genetically altered ES cells after transfection of the Nkx2.2 gene to determine their ability to differentiate into insulin-producing cells in vitro.
To assess the effect of the exogenous Nkx2.2 transgene on generation of insulin-producing ES-derived cells, we considered that an EB-based 1-step culture protocol[11,15,23] that supported natural differentiation of ES cells would be better than an EB-based multi-step protocol[24] that directed an oriented differentiation of ES cells toward pancreatic cell lineages using serum-free culture followed by a combination of growth factors. Since a promoted induction of insulin-producing cells was expected, a less efficient differentiation protocol was better suited to demonstrate the promoted action than a more sophisticated and efficient protocol. In addition, some investigators have speculated that the insulin-immuno-positive nature of cultured cells derived in an EB-based multi-step method is due to the incorporation of insulin from the culture medium[25,26], while it is also suspected that such insulin-incorporated cells are apoptotic. Therefore, in the present study, EBs are simply allowed to attach and grow in gelatin-coated dishes in media containing FCS without growth factors, including insulin.
Since the emergence of insulin-producing cells among the simple EB outgrowths was expected to be rare, we used DTZ to locate those that produced insulin. DTZ is a zinc-binding substance, and known to stain crimson red the pancreatic islets from such animals, as mice, dogs and pigs, as well as those from human beings . We previously reported that DTZ-stained cellular clusters appeared in EB outgrowths and that the clusters demonstrated characteristics similar to pancreatic islets[11]. In the present study, DTZ-stained cellular clusters appeared 2 wk earlier in the Nkx-ES-derived EB outgrowths, as compared to the wt-ES-derived EB outgrowths. Those cellular clusters were immunopositive for insulin and the presence of secreted insulin was confirmed by ELISA. Further, in addition to an early emergence, an increased frequency of DTZ-stained cells was also observed in the Nkx-ES-derived EB outgrowths. These results suggest that the fate of differentiating cells was favorably affected toward the generation of insulin-producing cells.
In spite of our success, the DTZ-positive cellular fraction still accounted for only 1% of the differentiated Nkx-ES cells. To achieve a more efficient generation of insulin- producing cells from ES cells in vitro, controlled regulation of other transcriptional factors that are involved in pancreatic β-cell differentiation might be necessary. Indeed, a highly efficient production of insulin-producing cells from ES cells has been reported by the expression of exogenously transfected pdx-1, pax 4, or Nkx6.1[27-29].
The precise mechanisms by which Nkx-ES cells promote the induction of insulin-producing cells is unknown, thus sequential analyses of a number of transcription factors that are involved in pancreatic β-cell differentiation must be examined in differentiating Nkx-ES cells. Although we have not performed such an analysis, an important finding to investigate is the early expression of insulin seen in the present differentiating Nkx-ES cells. Nkx2.2 expression preceded insulin expression in differentiating wt-ES cells (Figure 3A), which has also been seen in the pancreatic β-cell development[30]. Therefore, Nkx-ES cells may directly skip to that process following Nkx2.2 expression. Glucose-dependent insulin secretion was not observed in the cultures of ES-derived insulin-secreting cells, which we considered may have been due to the high concentration of glucose present in the culture media, in which the ES cells were allowed to differentiate until the insulin detection assay.
In summary, we generated gene-engineered Nkx2.2-expressing ES cells and demonstrated their ability to induce insulin-producing cells in vitro. Our results show that gene-engineered ES may be a useful source of insulin-producing cells.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3996] [Cited by in RCA: 3827] [Article Influence: 153.1] [Reference Citation Analysis (0)] |
| 2. | Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5956] [Cited by in RCA: 5428] [Article Influence: 123.4] [Reference Citation Analysis (0)] |
| 3. | Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634-7638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3879] [Cited by in RCA: 3581] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 4. | Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 576] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 5. | Hori Y, Rulifson IC, Tsai BC, Heit JJ, Cahoy JD, Kim SK. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:16105-16110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 292] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 6. | Kahan BW, Jacobson LM, Hullett DA, Ochoada JM, Oberley TD, Lang KM, Odorico JS. Pancreatic precursors and differentiated islet cell types from murine embryonic stem cells: an in vitro model to study islet differentiation. Diabetes. 2003;52:2016-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Moritoh Y, Yamato E, Yasui Y, Miyazaki S, Miyazaki J. Analysis of insulin-producing cells during in vitro differentiation from feeder-free embryonic stem cells. Diabetes. 2003;52:1163-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Segev H, Fishman B, Ziskind A, Shulman M, Itskovitz-Eldor J. Differentiation of human embryonic stem cells into insulin-producing clusters. Stem Cells. 2004;22:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 225] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 9. | Blyszczuk P, Wobus AM. Stem cells and pancreatic differentiation in vitro. J Biotechnol. 2004;113:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Lester LB, Kuo HC, Andrews L, Nauert B, Wolf DP. Directed differentiation of rhesus monkey ES cells into pancreatic cell phenotypes. Reprod Biol Endocrinol. 2004;2:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Shiroi A, Yoshikawa M, Yokota H, Fukui H, Ishizaka S, Tatsumi K, Takahashi Y. Identification of insulin-producing cells derived from embryonic stem cells by zinc-chelating dithizone. Stem Cells. 2002;20:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533-3542. [PubMed] |
| 13. | Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Schwitzgebel V, Hayes-Jordan A, German M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533-5540. [PubMed] |
| 14. | Sussel L, Kalamaras J, Hartigan-O'Connor DJ, Meneses JJ, Pedersen RA, Rubenstein JL, German MS. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213-2221. [PubMed] |
| 15. | Yamada T, Yoshikawa M, Takaki M, Torihashi S, Kato Y, Nakajima Y, Ishizaka S, Tsunoda Y. In vitro functional gut-like organ formation from mouse embryonic stem cells. Stem Cells. 2002;20:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Hooper M, Hardy K, Handyside A, Hunter S, Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987;326:292-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 892] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 17. | Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2725] [Cited by in RCA: 2668] [Article Influence: 106.7] [Reference Citation Analysis (0)] |
| 18. | Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1161] [Cited by in RCA: 1163] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 19. | Latif ZA, Noel J, Alejandro R. A simple method of staining fresh and cultured islets. Transplantation. 1988;45:827-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 210] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Clark SA, Borland KM, Sherman SD, Rusack TC, Chick WL. Staining and in vitro toxicity of dithizone with canine, porcine, and bovine islets. Cell Transplant. 1994;3:299-306. [PubMed] |
| 21. | Shewade YM, Umrani M, Bhonde RR. Large-scale isolation of islets by tissue culture of adult mouse pancreas. Transplant Proc. 1999;31:1721-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Watada H, Scheel DW, Leung J, German MS. Distinct gene expression programs function in progenitor and mature islet cells. J Biol Chem. 2003;278:17130-17140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Yamada T, Yoshikawa M, Kanda S, Kato Y, Nakajima Y, Ishizaka S, Tsunoda Y. In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells. 2002;20:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 211] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 970] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 25. | Rajagopal J, Anderson WJ, Kume S, Martinez OI, Melton DA. Insulin staining of ES cell progeny from insulin uptake. Science. 2003;299:363. [PubMed] |
| 26. | Sipione S, Eshpeter A, Lyon JG, Korbutt GS, Bleackley RC. Insulin expressing cells from differentiated embryonic stem cells are not beta cells. Diabetologia. 2004;47:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Miyazaki S, Yamato E, Miyazaki J. Regulated expression of pdx-1 promotes in vitro differentiation of insulin-producing cells from embryonic stem cells. Diabetes. 2004;53:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, Wobus AM. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci USA. 2003;100:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 297] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 29. | León-Quinto T, Jones J, Skoudy A, Burcin M, Soria B. In vitro directed differentiation of mouse embryonic stem cells into insulin-producing cells. Diabetologia. 2004;47:1442-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Soria B. In-vitro differentiation of pancreatic beta-cells. Differentiation. 2001;68:205-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |