Published online Jul 21, 2005. doi: 10.3748/wjg.v11.i27.4148
Revised: November 23, 2004
Accepted: November 29, 2004
Published online: July 21, 2005
AIM: Ghrelin is an endogenous ligand for the growth hormone secretagogue receptor, and it plays a role in stimulating the growth hormone secretion, food intake, body weight gain and gastric motility. Eradication of
Helicobacter pylori (H pylori) was shown to be associated with increase of the body weight. On the other hand, H pylori infection evokes the release of gastric IL-1β. The present study was designed to investigate the involvement of the gastric IL-1 signal in the ghrelin dynamics in H pylori-colonized mice.
METHODS: Twelve-week-old female IL-1-receptor type 1-homozygous-knockout mice (IL-1R1-/-) and their wild-type littermates (WT) were orally inoculated with H pylori (Hp group), while other cohorts received oral inoculation of culture medium (Cont group). Thirteen weeks after the inoculation, the mice were examined. The plasma and stomach ghrelin levels and the gastric preproghrelin mRNA were measured.
RESULTS: Although the WT mice with H pylori infection showed a significantly decreased body weight as compared with that of the animals without H pylori infection, H pylori infection did not influence the body weight of the IL-1R1-knockout (IL-1R1-/-) mice. In the H pylori-infected IL-1R1-/- mice, the total and active ghrelin levels in the plasma were significantly increased, and the gastric ghrelin level was decreased. No significant differences were noted in the gastric preproghrelin mRNA expression.
CONCLUSION: Ghrelin secretion triggered by H pylori infection might be suppressed by IL-1β, the release of which is also induced by the infection, resulting in the body weight loss of mice with H pylori infection.
-
Citation: Abiko Y, Suzuki H, Masaoka T, Nomura S, Kurabayashi K, Hosoda H, Kangawa K, Hibi T. Enhanced plasma ghrelin levels in
Helicobacter pylori -colonized, interleukin-1-receptor type 1-homozygous knockout (IL-1R1-/-) mice. World J Gastroenterol 2005; 11(27): 4148-4153 - URL: https://www.wjgnet.com/1007-9327/full/v11/i27/4148.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i27.4148
Ghrelin, an endogenous ligand for the growth hormone secretagogue receptor (GHS-R), stimulates growth hormone (GH) release from cultured pituitary cells in a dose-dependent manner[1], and is produced and secreted from the A-like cells found mainly in the oxyntic glands of the gastric fundus[2]. Ghrelin is now known to play a role in not only GH release, but also in controlling the appetite and body weight. Since both parenterally and intracerebro-ventricularly administered ghrelin have been shown to stimulate food intake and increase the body weight of mice and rats with free access to food, even those animals with GH deficiency[3,4], the control of appetite and body weight may be independent of GH release. Ghrelin, a 28-amino-acid peptide, is activated when its third serine residue is acylated by n-octanoic acid, and GHS-R is responsive to the first four or five residues including the octanylated serine residue of the whole ghrelin peptide[5]. GHS-R has been shown to be present in the pituitary, hypothalamus, adrenal glands, thyroid, pancreas, myocardium, spleen and testes[1,6-8]. Ghrelin stimulates the expression of both NPY and AGRP mRNA in the hypothalamus. The central orexigenic effect of ghrelin is mediated by the NPY/AGRP-expressing neurons in the hypothalamus[3,9-11]. On the other hand, ghrelin has also been reported to suppress vagal afferent activity[2]. The peripheral orexigenic effect of ghrelin may be mediated, at least in part, by its suppressive effect on the vagal afferent activity.
IL-1β is a pro-inflammatory cytokine that mediates the cachectic process by stimulating the expression and release of leptin[12] and/or by mimicking the effect on the hypothalamus of excessive negative-feedback signaling from leptin[13]. Cachexia is a condition characterized by wasting, emaciation, feebleness and inanition. It was recently reported that the levels of both ghrelin peptide and ghrelin mRNA in the stomach were up-regulated in a mouse model of cancer cachexia[14]. In cachectic mice with increased plasma levels of IL-1β, the plasma concentrations of ghrelin also increased with the progression of cachexia[15]. This result suggests that a close relationship might exist between the ghrelin dynamics and the cachectic process mediated by IL-1. IL-1β is an anorexigenic substance, just like CCK, leptin, gastrin-related protein and bombesin, and antagonizes the actions of ghrelin. Asakawa et al reported that parenterally administered IL-1β decreased NPY mRNA expression in the hypothalamus and preproghrelin mRNA expression in the stomach, and that intraperitoneally administered ghrelin inhibited the severity of IL-1β-induced anorexia.
Helicobacter pylori (H pylori) infection is known to be a major pathogenetic factor in the development of gastritis, peptic ulcer disease and gastric malignancy[16,17]. Attachment of H pylori to the gastric mucosa induces inflammation, which is associated with the release of various cytokines, including IL-1β[18]. Although the importance of the anorexigenic effect of IL-1β in cases with H pylori infection has not yet been clarified, it has been observed clinically that H pylori eradication is often followed by improvement of some nutritional parameters, such as the body weight and the serum levels of total cholesterol, total protein and albumin[19]. We recently reported that H pylori infection could modify the plasma and gastric ghrelin dynamics in Mongolian gerbils[20]. In humans, however, H pylori infection has been reported not to be associated with any changes of the plasma ghrelin levels[21,22], although eradication of H pylori has been shown by some to be associated with increases of the plasma ghrelin levels[23]. Therefore, regulation of the ghrelin dynamics and its influence on the body weight control in cases with H pylori infection still remains to be clarified.
We designed the present study to investigate the role of the gastric IL-1 signal in the regulation of the ghrelin dynamics and its influence on the body weight control under in cases with H pylori infection.
All experiments and procedures carried out on the animals were approved by the Keio University Animal Research Committee (No. 023009). Twelve-week-old, specific-pathogen-free female interleukin-1 (IL-1)-receptor type 1-homozygous-knockout mice (IL-1R1-/-) on a C57BL/6 background (The Jackson Lab., Bar Harbor, ME, USA) and their wild-type cohorts as controls (WT) were used for the study. The Sydney Strain of H pylori (SS1) was grown from frozen stocks at 37°C under microaerobic conditions for 24-36 h in lysed horse-blood agar supplemented with antibiotics, harvested in Brucella broth, and administered to the mice immediately after being harvested. Six IL-1R1-/-mice and five WT mice were orally inoculated with H pylori (1.5×108 CFU/mL, 0.5 mL), while another six IL-1R1-/-mice and five WT mice were orally inoculated with the culture medium alone as control. A week later, each animal was inoculated again with the aforementioned bacterial strain. Thirteen weeks after the first inoculation, all the mice were examined under ether anesthesia. The mice were then deprived of food for 17 h before being killed. The body weight of each mouse was measured just before the examination.
H pylori infection was diagnosed by determining the number of CFUs in a microaerobic bacterial culture. Briefly, the diluted homogenates of the stomachs were plated on to Brucella agar plates containing 10% horse blood, 2.5 µg/mL amphotericin B, 9 µg/mL vancomycin, 0.32 µg/mL polymyxin B, 5 µg/mL trimethoprim, and 50 µg/mL 2, 3, 5-triphenyl-tetrazolium chloride. The plates were then incubated at 37°C in a microaerobic atmosphere for 7 d[24]. All of the H pylori inoculated animals were confirmed as H pylori positive by this procedure in the present study.
Whole-blood samples were obtained from the right ventricle in tubes containing EDTA-2Na (1 mg/mL blood) and aprotinin (500 kIU/mL blood). The tubes were centrifuged and the plasma samples were stored at -80°C.
Plasma total ghrelin was measured using the Desacyl-Ghrelin ELISA Kit (Mitsubishi Kagaku Medical, Inc., Ibaraki, Japan), and plasma active ghrelin was measured using the Active Ghrelin ELISA Kit (Mitsubishi Kagaku Medical, Inc., Ibaraki, Japan). Total ghrelin, i.e., active plus inactive ghrelin, was measured using an antibody directed against the C-terminal end (residues 1 to 11) of ghrelin, while an antibody directed against the N-terminal end of ghrelin (residues 13 to 28) was used for the measurement of active ghrelin. The plasma samples were placed in testing wells coated with the ghrelin antibodies. Then, the plate with the wells was incubated for 2 h at room temperature. After incubation, the samples were washed and diluted HRP was added to the wells. The plate was then incubated again for 1 h at room temperature. The samples were washed and substrate solution was added to the wells. The plate was incubated for a further 30 min at room temperature under shade conditions. After the reaction, a reagent was added to stop the reaction, and the absorbance of each well was measured at 450 nm.
Immediately after the mice were sacrificed, the stomachs of the animals were removed and opened along the greater curvature. Tissue samples of the gastric mucosa were collected in tubes containing PBS and protease inhibitors (100 µmol/L phenylmethylsulfonyl fluoride, 10 µg/mL aprotinin) and sonicated over ice in 30 consecutive 0.5 s bursts at 0.5 s intervals, at a power setting of 150 W (VCX 50; Sonics and Materials, Inc., Newton, CT, USA). The total protein content in the homogenates was measured by modified Lowry’s method[25], as described by Smith et al[26].
Myeloperoxidase (MPO) activity, as an index of tissue-associated neutrophil accumulation, was determined by a modification of Grisham et al’s method[27]. Aliquots containing 100 µL of the mucosal homogenates were centrifuged at 8 000 r/min for 15 min at 4°C to separate the pellets of insoluble cellular debris. The pellets were rehomogenized in an equal volume of 0.05 mol/L potassium phosphate buffer (pH 5.4) containing 0.5%-hexadecyltrime-thylammonium bromide. The samples were then centrifuged at 8 000 r/min for 15 min at 4°C and the supernatants were reserved. The MPO activity in the supernatants was assessed by measuring the H2O2-dependent oxidation of 3, 3’, 5, 5’-tetramethylbenzidine. One unit of enzyme activity was defined as the amount of MPO that caused a change in the absorbance of 1.0/min at 655 nm, at 25°C.
Fresh whole-anterior-wall specimens of the glandular stomach were frozen immediately after collection in liquid nitrogen and stored at -80°C. Each sample was boiled for five min in a 10-fold volume of water to inactivate the intrinsic proteases. The solution was adjusted to 1N acetic acid by addition of 180 microliter acetic acid after cooling, and the tissue was homogenized. The supernatant was lyophilized and subjected to RIA to measure the ghrelin level. The extraction efficiency of tissue ghrelin was more than 95%.
Two RIA techniques were used for measuring ghrelin, as described previously[28]. Briefly, ghrelin levels were measured using two polyclonal rabbit antibodies raised against an N terminal (1-11) (Gly1-Lys11) or C terminal (13-28) (Gln13-Arg28) fragment of rat ghrelin. Two tracer ligands were synthesized: [Tyr29]-rat ghrelin for antirat ghrelin (1-11) antiserum and [Tyr12]-rat ghrelin [13-28] for antirat ghrelin [13-28] antiserum. The RIA incubation mixtures containing 100 µL of either standard ghrelin or unknown samples containing 200 µL of antiserum diluted in RIA buffer containing 0.5% normal rabbit serum, were initially incubated for 12 h. Then, the mixture was incubated for 36 h after the addition of 100 µL of 125I labeled tracer (15 000 cpm). Antirabbit IgG goat serum (100 µL) was added followed by incubation for an additional 24 h. Free and bound tracers were then separated by centrifugation at 3 000 r/min for 30 min. Following aspiration of the supernatant, the radioactivity in the pellet was quantified using a gamma counter (ARC-600; Aloka, Tokyo, Japan). All assays were performed at 4°C. The antirat ghrelin (1-11) antiseurm specifically recognized the n-octanoylated form of rat ghrelin, but not the des-acyl form. The antirat ghrelin (13-28) antiserum recognized both the acylated and des-acyl forms of rat ghrelin equally efficiently. Both antisera were equally cross-reactive with human and gerbil ghrelin, and did not recognize the other enteric peptides. The respective intra- and interassay coefficients of variation for the N terminal RIA were 3% and 6%, and for the C terminal RIA were 6% and 9%.
Total RNA was extracted from the stomach of the mice using the RNeasy Mini Kit (Qiagen). A TaqMan quantitative real-time RT-PCR was performed to detect preproghrelin mRNA and GAPDH mRNA using the ABI PRISM 7700 sequence detection system (PE Applied Biosystems)[29].
The following primers were used to amplify preproghrelin mRNA: forward-ghrelin (5’-GGA ATC CAA GAA GCC ACC AGC-3’), reverse-ghrelin (5’-GCT CCT GAC AGC TTG ATG CCA-3’), and Taqman-ghrelin (5’-FAM-AAC TGC AGC CAC GAG CTC TGG AAG GC-TAMRA-3’). The following primers were used to amplify GAPDH mRNA as the internal control: forward primer (5’-TTC AAC GGC ACA GTC AAG GC-3’), reverse primer (5’-GCC TTC TCC ATG GTG GTG AAG-3’), and Taqman probe (5’-FAM-CCC ATC ACC ATC TTC CAG GAG CGA GA-TAMRA-3’).
The preproghrelin mRNA expression levels were normalized using the GAPDH mRNA expression levels.
All the data were expressed as the mean±SE. The data were analyzed using one-way analysis of variance, followed by Scheffe’s multiple comparison tests. A value of P < 0.05 was considered to denote statistical significance.
Among the WT mice, the H pylori-infected mice showed a significantly reduced body weight as compared to the uninfected and control mice (P < 0.001). Although the basic body weight of the IL-1R1-/- mice was lower than that of the WT mice, no further reduction of the body weight of these mice was observed after H pylori infection (Table 1).
| Wild type mice (g) | IL-1R1-/-/- mice (g) | ||
| Not-infected | Infected | Not-infected | Infected |
| 26.0 ± 0.71 | 22.1 ± 0.54b | 23.4 ± 0.51 | 23.1 ± 0.34 |
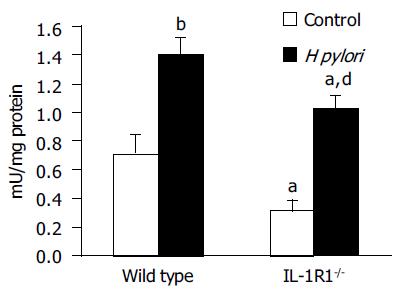
H pylori infection increased the MPO activity in the gastric mucosa in both the WT (P < 0.01) and IL-1R1-/- (P < 0.01) mice, although in the IL-1R1-/- mice, the increase was significantly less marked (P < 0.05) (Figure 1).
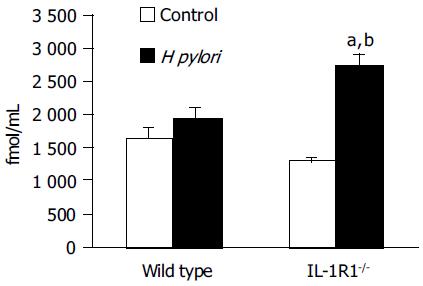
Among the WT mice, the H pylori-infected animals showed no significant increase of the plasma total ghrelin levels as compared to the uninfected and control mice. In the case of the IL-1R1-/- mice, although simple lack of the IL-1 signal did not affect the plasma total ghrelin dynamics, H pylori infection of these mice was associated with a marked increase of the plasma total ghrelin levels (P < 0.05; compared with the uninfected mice, P < 0.001; compared with the wild-type mice) (Figure 2).
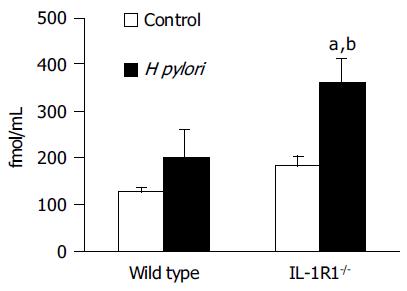
The same tendency was shown for the plasma active ghrelin dynamics. Among the WT mice, the H pylori-infected mice showed a tendency towards increase in the plasma active ghrelin levels as compared to the uninfected and control mice, but the increase was not statistically significant. On the other hand, in the IL-1R1-/- mice, while a simple lack of the IL-1 signal did not affect the plasma active ghrelin dynamics, H pylori infection of these animals was associated with a marked increase of the plasma active ghrelin levels (P < 0.01; compared with the uninfected mice, P < 0.05; compared with the wild-type mice) (Figure 3).
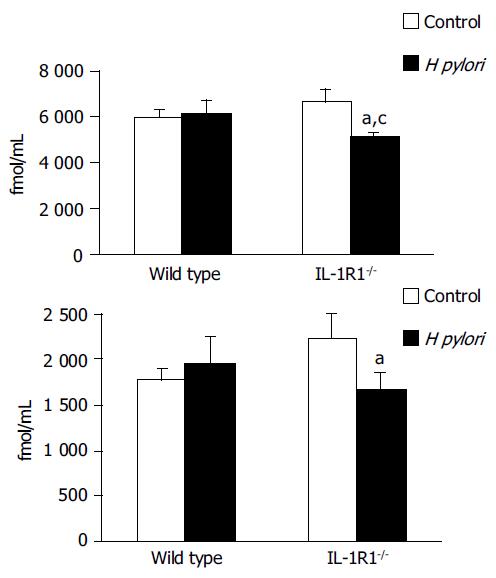
In contrast to the effects of H pylori on the plasma ghrelin dynamics, the ghrelin levels in the stomach, both total and active, were significantly decreased in the H pylori-infected IL-1R1-/- mice (P < 0.05) (Figure 4A and B), indicating that ghrelin is secreted into the blood from its stores in the stomach.
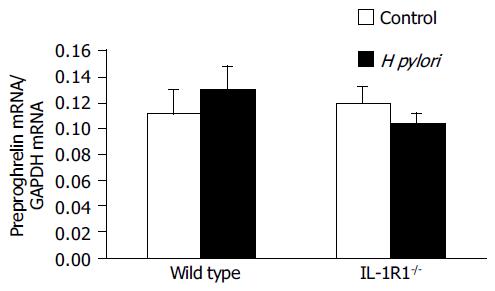
Preproghrelin mRNA expression, evaluated in comparison with the expression of GAPDH mRNA, was not modified by either H pylori infection or lack of the IL-1 signal, or both (Figure 5). These results suggest that the stomach ghrelin stores were not replenished after ghrelin secretion into the blood.
It is widely known that H pylori infection is associated with loss of body weight. In the present study, although H pylori-infected wild-type (WT) mice showed body weight loss, no such weight loss was observed in IL-1R1-/- mice with H pylori infection (Table 1). On the other hand, although an increase in the level of gastric mucosal neutrophil accumulation was noted in both WT and IL-1R1-/- mice with H pylori infection, the neutrophil accumulation was significantly less marked in H pylori-infected IL-1R1 -/- mice than in H pylori-infected WT mice (Figure 1), indicating that the lack of the IL-1 signal could affect the regulatory mechanisms of both body weight and gastric mucosal inflammation.
It has previously been reported that the administration of lipopolysaccharide (LPS) is associated with a reduction in the plasma levels of ghrelin[30,31] and increase in the plasma levels of IL-1β[31]. According to the study by Burgess et al[32], while intracerebroventricular administration of LPS greatly reduced the food intake in WT mice, no such effect was observed in mice deficient in the IL-1 β-converting enzyme. In addition, as mentioned above, it has been reported that injection of IL-1β was followed by a decrease in the gastric preproghrelin mRNA expression[9]. These results suggest that the anorexigenic effect of LPS may be mediated by IL-1β and ghrelin, a downstream molecule in the cascade. The production of IL-1β might be enhanced by LPS and the release of ghrelin might be suppressed by IL-1 β.
We recently reported from a study in Mongolian gerbils, that the plasma levels of ghrelin are enhanced for a restricted period after H pylori inoculation[20]. In the present study, while the plasma levels of ghrelin showed no significant increase in the WT mice with H pylori infection, the levels were significantly elevated in IL-1R1-/- mice with H pylori infection (Figure 2, 3), indicating that IL-1 may suppress ghrelin secretion, as mentioned above. As in Mongolian gerbils, H pylori infection also stimulates ghrelin secretion in mice, but the secretion is suppressed by IL-1 β, a mediator whose production is also enhanced by H pylori infection. Therefore, in IL-1-signal-deficient mice with H pylori infection, ghrelin production and release into the blood occurs unopposed by the inhibitory action of IL-1 on these processes.
Contrary to the observations on the ghrelin dynamics, the gastric expression of leptin mRNA, one of the important anorexigenic peptides produced by adipocytes, is significantly increased by H pylori infection[33]; expression of this mRNA is also significantly enhanced by IL-1 administration[12]. The central pathway of appetite control by ghrelin is reported to be mediated by neuropeptide Y (NPY)-expressing neurons in the hypothalamus. Ghrelin increases the activity of NPY-expressing neurons, and hence food intake[9,10]. The same NPY-expressing neurons in the hypothalamus have also been reported to be involved in the suppression of appetite by leptin. Leptin decreases the mRNA expression of NPY in the neurons of the hypothalamus[11], thereby suppressing food intake and causing loss of body weight[3,11]. The effects of ghrelin and leptin are considered to be competitively regulated by the hypothalamic NPY-expressing neurons.
Thus, in the WT mice, H pylori infection stimulates both ghrelin secretion and IL-1β release, but excessive ghrelin secretion is suppressed by IL-1β, whereas leptin secretion, also stimulated by H pylori, is promoted by IL-1β. The competitive actions of ghrelin and leptin in the hypothalamic NPY-expressing neurons result in a decrease of the appetite and body weight of WT mice with H pylori infection. On the other hand, in the H pylori-infected IL-1R1-/- mice, the inhibitory effect of the IL-1 signal on ghrelin secretion is absent, and the enhanced ghrelin secretion combined with the enhanced leptin secretion might result in a dominant effect of ghrelin on the appetite control, and body weight loss does not become manifest in these animals.
While the secretion of stored gastric ghrelin is affected by H pylori, the gastric preproghrelin mRNA expression was not significantly enhanced by H pylori infection in the present study (Figure 5). While in Mongolian gerbils, H pylori infection was associated with increased plasma ghrelin levels and decreased gastric ghrelin and preproghrelin mRNA expression[20], the infection in mice had no significant effect on any of the above three parameters. According to Asakawa et al[9], IL-1 administration reduced gastric preproghrelin mRNA expression; this may be the reason why the IL-1-receptor-knockout mice in the present study did not show any changes of the gastric preproghrelin mRNA expression. Thus, there may be species differences in the hormone dynamics and their reactivity to stimulation, such as in the event of H pylori infection.
In conclusion, ghrelin secretion triggered by H pylori infection might be suppressed by IL-1β, resulting in body weight loss of the infected mice.
A part of the present data was presented in the Topic Forum of Digestive Disease Week on May, 17th, 2004, in New Orleans, USA.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5961] [Cited by in RCA: 5889] [Article Influence: 226.5] [Reference Citation Analysis (0)] |
| 2. | Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 805] [Cited by in RCA: 771] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 3. | Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2466] [Cited by in RCA: 2457] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 4. | Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325-4328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 834] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 5. | Bednarek MA, Feighner SD, Pong SS, McKee KK, Hreniuk DL, Silva MV, Warren VA, Howard AD, Van Der Ploeg LH, Heck JV. Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem. 2000;43:4370-4376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 428] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 6. | Gaytan F, Barreiro ML, Caminos JE, Chopin LK, Herington AC, Morales C, Pinilla L, Paniagua R, Nistal M, Casanueva FF. Expression of ghrelin and its functional receptor, the type 1a growth hormone secretagogue receptor, in normal human testis and testicular tumors. J Clin Endocrinol Metab. 2004;89:400-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 841] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 8. | Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 757] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 9. | Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 843] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 10. | Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology. 2000;141:4797-4800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 193] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, Hayashi T, Inoue G, Hosoda K, Kojima M. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 569] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 12. | Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, Flier JS, Lowell BB, Fraker DL, Alexander HR. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 568] [Cited by in RCA: 557] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 13. | Inui A. Cancer anorexia-cachexia syndrome: are neuropeptides the key? Cancer Res. 1999;59:4493-4501. [PubMed] |
| 14. | Hanada T, Toshinai K, Kajimura N, Nara-Ashizawa N, Tsukada T, Hayashi Y, Osuye K, Kangawa K, Matsukura S, Nakazato M. Anti-cachectic effect of ghrelin in nude mice bearing human melanoma cells. Biochem Biophys Res Commun. 2003;301:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Hanada T, Toshinai K, Date Y, Kajimura N, Tsukada T, Hayashi Y, Kangawa K, Nakazato M. Upregulation of ghrelin expression in cachectic nude mice bearing human melanoma cells. Metabolism. 2004;53:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3302] [Cited by in RCA: 3263] [Article Influence: 79.6] [Reference Citation Analysis (1)] |
| 17. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3183] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 18. | Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut. 1997;41:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 281] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Furuta T, Shirai N, Xiao F, Takashima M, Hanai H. Effect of Helicobacter pylori infection and its eradication on nutrition. Aliment Pharmacol Ther. 2002;16:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Suzuki H, Masaoka T, Hosoda H, Ota T, Minegishi Y, Nomura S, Kangawa K, Ishii H. Helicobacter pylori infection modifies gastric and plasma ghrelin dynamics in Mongolian gerbils. Gut. 2004;53:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Gokcel A, Gumurdulu Y, Kayaselcuk F, Serin E, Ozer B, Ozsahin AK, Guvener N. Helicobacter pylori has no effect on plasma ghrelin levels. Eur J Endocrinol. 2003;148:423-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Suzuki H, Masaoka T, Hosoda H, Nomura S, Ohara T, Kangawa K, Ishii H, Hibi T. Plasma ghrelin concentration correlates with the levels of serum pepsinogen I and pepsinogen I/II ratio--a possible novel and non-invasive marker for gastric atrophy. Hepatogastroenterology. 2004;51:1249-1254. [PubMed] |
| 23. | Nwokolo CU, Freshwater DA, O'Hare P, Randeva HS. Plasma ghrelin following cure of Helicobacter pylori. Gut. 2003;52:637-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Takahashi S, Keto Y, Fujita H, Muramatsu H, Nishino T, Okabe S. Pathological changes in the formation of Helicobacter pylori-induced gastric lesions in Mongolian gerbils. Dig Dis Sci. 1998;43:754-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 26. | Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16085] [Cited by in RCA: 16153] [Article Influence: 403.8] [Reference Citation Analysis (0)] |
| 27. | Grisham MB, Hernandez LA, Granger DN. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986;251:G567-G574. [PubMed] |
| 28. | Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279:909-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 653] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 29. | Masaoka T, Suzuki H, Hosoda H, Ota T, Minegishi Y, Nagata H, Kangawa K, Ishii H. Enhanced plasma ghrelin levels in rats with streptozotocin-induced diabetes. FEBS Lett. 2003;541:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Basa NR, Wang L, Arteaga JR, Heber D, Livingston EH, Taché Y. Bacterial lipopolysaccharide shifts fasted plasma ghrelin to postprandial levels in rats. Neurosci Lett. 2003;343:25-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Zuckerman SH, Shellhaas J, Butler LD. Differential regulation of lipopolysaccharide-induced interleukin 1 and tumor necrosis factor synthesis: effects of endogenous and exogenous glucocorticoids and the role of the pituitary-adrenal axis. Eur J Immunol. 1989;19:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 221] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Burgess W, Gheusi G, Yao J, Johnson RW, Dantzer R, Kelley KW. Interleukin-1beta-converting enzyme-deficient mice resist central but not systemic endotoxin-induced anorexia. Am J Physiol. 1998;274:R1829-R1833. [PubMed] |
| 33. | Azuma T, Suto H, Ito Y, Ohtani M, Dojo M, Kuriyama M, Kato T. Gastric leptin and Helicobacter pylori infection. Gut. 2001;49:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |