Published online Jul 21, 2005. doi: 10.3748/wjg.v11.i27.4140
Revised: November 20, 2004
Accepted: November 23, 2004
Published online: July 21, 2005
AIM: To assess long-term effects of Helicobacter pylori (H pylori) eradication on antral G cell morphology and function in patients with and without duodenal ulcer (DU).
METHODS: Consecutive dyspeptic patients referred to the endoscopy entered the study. Out of 39 H pylori positive patients, 8 had DU (H pylori +DU) and 31 gastritis (H pylori +G). Control groups consisted of 11 uninfected dyspeptic patients (CG1) and 7 healthy volunteers (CG2). Basal plasma gastrin (PGL), antral tissue gastrin concentrations (ATGC), immunohistochemical and electron microscopic characteristics of G cells were determined, prior to and 6 mo after therapy.
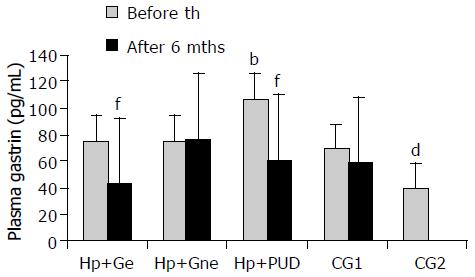
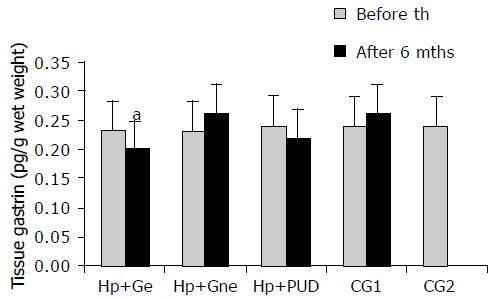
RESULTS: We demonstrated elevated PGL in infected patients compared to uninfected controls prior to therapy. Elevated PGL were registered in all H pylori+patients (H pylori +DU: 106.78 ± 22.72 pg/mL, H pylori +G: 74.95 ± 15.63, CG1: 68.59 ± 17.97, CG2: 39.24 ± 5.59 pg/mL, P < 0.01). Successful eradication (e) therapy in H pylori+patients lead to significant decrease in PGL (H pylori+DU: 59.93 ± 9.40 and H pylori +Ge: 42.36 ± 10.28 pg/mL, P < 0.001). ATGC at the beginning of the study were similar in infected and uninfected patients and eradication therapy lead to significant decrease in ATGC in H pylori +gastritis, but not in DU patients. In the H pylori +DU patients, the mean number of antral G cells was significantly lower in comparison with all other groups (P < 0.01), but after successful eradication was close to normal values found in controls. By contrast, G cell number and volume density were significantly decreased (P < 0.01) in H pylori +Ge group after successful eradication therapy (294 ± 32 and 0.31 ± 0.02, respectively), in comparison to values before eradication (416 ± 40 and 0.48 ± 0.09). No significant change of the G cell/total endocrine cell ratio was observed during the 6 mo of follow up in any of the groups. A reversible increase in G cell secretory function was seen in all infected individuals, demonstrated by a more prominent secretory apparatus. However, differences between DU and gastritis group were identified.
CONCLUSION: H pylori infection induces antral G cell hyperfunction resulting in increased gastrin synthesis and secretion. After eradication therapy complete morphological and functional recovery is observed in patients with gastritis. In the DU patients some other factors unrelated to the H pylori infection influence antral G cell morphology and function.
-
Citation: Sokic-Milutinovic A, Todorovic V, Milosavljevic T, Micev M, Drndarevic N, Mitrovic O. Gastrin and antral G cells in course of
Helicobacter pylori eradication: Six months follow up study. World J Gastroenterol 2005; 11(27): 4140-4147 - URL: https://www.wjgnet.com/1007-9327/full/v11/i27/4140.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i27.4140
Helicobacter pylori (H pylori) causes chronic active type B gastritis and is involved in the pathogenesis of peptic ulcer disease (PUD) and gastric cancer[1]. PUD symptoms were almost immediately attributed to the presence of H pylori infection, however a positive correlation between the infection and non-ulcer dyspepsia (NUD) was acknowledged only after the results of meta-analysis[2]. NUD should be considered in dyspeptic patients when symptoms persist for at least a month and endoscopy reveals neither peptic ulcer nor signs of gastric cancer[3].
Gastrin is a secretory product of antral and duodenal G cells. Two main forms of gastrin (gastrin-17 and -34) are present in the circulation. About 95% of antral gastrin is gastrin-17[3,4] . Stimuli for gastrin secretion are well identified and include food intake, presence of digested amino acids in the lumen, cholinergic stimuli, and antral alkalization[3,4].
Although elevated serum gastrin levels are frequently observed in individuals with chronic H pylori infection[5-7], its pathophysiological significance in gastric mucosal inflammation remains unclear. Gastrin is capable of up-regulating CXC chemokines in gastric epithelial cells and therefore may contribute to the progression of the inflammatory process in the stomach[8]. Chronic hypergastrinemia is also associated with gastric argirophil cell hyperplasia in rats and humans and carcinoid tumor in Mongolian gerbils[9]. In the antrum of H pylori infected gerbils and humans enhanced apoptosis is an early and transient cell cycle event whilst epithelial cell proliferation peaks later and is related to increased gastrin levels. Based on these findings it was suggested that gastrin-dependent mechanism might be responsible for epithelial cell growth in H pylori colonized gastric mucosa[10,11]. It can be assumed that restored antral G cell function after H pylori eradication resulting in lower basal and meal-stimulated gastrin release would be a desirable event in both chronic gastritis and PUD. Most studies conducted so far focused on the effects of H pylori infection on gastric endocrine cells in patients with duodenal ulcer and came to different conclusions[5,6]. There have been few reports concerning G cell morphology[12-14] and/or gastrin secretion[7] in patients with gastritis.
The aim of our study was to investigate changes in antral G cell morphology and function in dyspeptic patients with H pylori infection and its possible restoration in course of eradication therapy.
We conducted an outpatient based prospective study in the Clinic for Gastroenterology and Hepatology, Clinical Center of Serbia, lasting for 6 mo after patients completed eradication therapy. Fifty consecutive dyspeptic patients referred to the endoscopy and seven healthy asymptomatic volunteers entered the study. Out of 39 H pylori positive patients had 31 histological signs of gastritis- H pylori +G and 8 DU- H pylori +DU. Control group 1 (CG1) consisted of 11 H pylori negative dyspeptic patients while 7 healthy asymptomatic volunteers were assigned to the control group 2 (CG2). All patients gave informed consent and the study protocol was approved by the local Ethics Committee. Mean age was 48 ± 15 years (28 males and 22 females), 21 were smokers and 21 had personal history of PUD. Exclusion criteria were in concordance with the recommendations from European H pylori Study Group[15]. H pylori infection was diagnosed by rapid urease test (RUT), histology and serology. A patient was defined as H pylori positive if histology and at least one of the other applied diagnostic methods were positive.
Routine endoscopy and biopsy samples Upper endoscopy was performed before therapy in all dyspeptic patients and repeated 6 mo after appropriate therapy. In healthy volunteers (CG2) endoscopy was performed only once. During endoscopy antral biopsy specimens, intended for routine histology, RUT test, determination of tissue gastrin levels, immunohistochemistry and electron microscopy were taken.
H pylori serology Blood samples were taken from the patients after endoscopic examination and sera were separated by centrifugation and stored at -20 ¡æ until analyzed. The concentration of anti- H pylori IgG antibodies was analyzed using the Pyloriset EIA- G IIITM (Orion Diagnostica, Finland), according to the manufacturers’ instructions.
Therapy A triple eradication therapy consisting of omeprazole 20 mg twice a day (bid), amoxicillin 1 000 mg b.i.d. and metronidazole 500 mg b.i.d. was administered for 7 d in patients with H pylori infection. In uninfected patients, symptomatic therapy consisting of antacids, H2 antagonists or proton pump inhibitors (PPIs) was prescribed for 2 wks. In the course of H pylori eradication, the disappearance of spiral bacteria from antral and corpus gastric mucosa, according to a negative RUT and histological examination, were observed.
Histological assessment Biopsies from antral and corpus mucosa were stained using hematoxylin- eosin and modified Giemsa staining procedure. Biopsy specimens were assessed according to the Sydney System[16,17] by a single, experienced pathologist who was blinded to the clinical presentation, endoscopic data and RUT results of the patient.
Plasma preparation Full blood samples for estimating fasting plasma gastrin levels were placed in ice/chilled tubes (5 mL) containing EDTA (2 mg) and proteinase inhibitor (Trasylol, 2 500 KIU). Plasma was extracted using standard procedure and stored below -70 ¡æ until further analysis.
Tissue extract preparation Each antral mucosa biopsy was washed with saline solution, measured and placed in to the tube together with 1 mL of distilled water. Gastrin extraction was performed in water bath on 95 ¡æ for 10 min, supernatant collected and after cooling stored below -70 ¡æ until further analysis.
Radioimmunoassay procedure (RIA) Plasma and tissue gastrin was determined under basal conditions using RIA protocol provided by Affiniti (UK) with rabbit antihuman gastrin- 17 antiserum. Final dilutions were for plasma 1:100 000 and for the tissue 1:500 000. Intra-assay and inter-assay coefficients were 9.0 and 8.4 respectively. Sensitivity of the method was 1 pmol/L.
Immunohistochemistry Two antral biopsies from each patient were used for immunohistochemistry and light microscopic morphometry. These were fixed with 10% buffered formalin and embedded in paraffin in the usual manner. Only well-oriented antral mucosa biopsies that allowed assessment of the full mucosal thickness were studied. The sections were stained with different immunohistochemical methods in order to identify antral gastrin-producing cells and evaluate the ratio of antral G cells/all antral endocrine cells. Immunohistochemical staining for G cells was performed using rabbit anti human gastrin-17 (1:300 dilution, Code No A0568, DAKO A/S, Denmark) and peroxidase-labeled streptavidin biotin method (DAKO LSAB+/HRP, DAKO A/S, Denmark). In addition, sections were double immunostained with both polyclonal antibody to gastrin mentioned above and mAb to synaptohysin (1:50 dilution, Code No. A0010, DAKO A/S, Denmark), using DAKO EnVisionR double stain system (Code No. K1395). For double immunostaining, antisynaptophysin antibody was applied first with DAB as a chromogen, followed by antigastrin-17 antibody as the second antibody, with AEC as a chromogen. Negative controls were conjugated with normal horse serum[18].
Routine electron microscopy The biopsy specimens of antral mucosa were immediately placed in a mixture of 2% glutaraldehyde in 0.2 mol/L sodium cacodylate buffer, pH 7.4, and fixed in the same fixative for 20 h at 4 ¡æ. After postfixation for 1 h in 1% osmium tetroxide in cacodylate buffer, the specimens were dehydrated in graded ethanol and embedded in Eppon 812R, with mucosa surface perpendicular to the cutting surface. The blocks were sectioned with an LKB ultratome II. Ultra thin sections were double-stained with uranyl acetate and lead-citrate before examination in an Opton 109 electron microscope.
Morphometric analysis of G cells detected by immunohistochemistry Three 5-μm thick immunostained sections of antral mucosa at intervals of 50 μm were analyzed. Weibel multipurpose test system containing 42 points and 21 lines was used for evaluation of volume density and number of G cells[19,20]. The total number of G cells per mm2 of antral mucosa, as well as, ratio of antral G/total antral endocrine cells was calculated by examination of single or double immunostained sections. All sections were examined randomly by two histologists.
Morphometric analysis of G cells detected by electron microscopy G cells were identified as described previously[21]. Morphometric analysis was performed using the methods described previously[19,20]. Cell profile areas were estimated by drawings of G-cells using Camera Lucida attached to a Reichert microscope and analyzed with an image analyzing system (MOP 3 Video plan; Carl Zeiss)[19,20].
Each time mean and SD was calculated for results presentation. A two sample paired or unpaired Student’s t-test and Wilcoxon rank sum test and ANOVA were used. A P value less than 0.05 was considered significant.
Clinical and demographic characteristics of the patients are shown in Table 1. Out of 39 H pylori-positive patients eradication therapy was successful in 32 (82.1%). In all DU patients the infection was successfully treated, together with 24 patients with gastritis (H pylori +Ge). In seven patients with gastritis eradication therapy failed (H pylori +Gne). No significant difference in demographic and clinical data between H pylori+Gne and H pylori+Ge patients was observed.
| H pylori +Ge (n = 24) | H pylori+ Gne (n = 7) | H pylori +DU (n = 8) | CG1 (n = 11) | CG2 (n = 7) | P | |
| Age (yr) | 47 ± 13 | 48 ± 9 | 47 ± 23 | 47 ± 19 | 33 ± 11 | NS |
| Sex (males) | 13 | 1 | 2 | 5 | 2 | NS |
| Smokers | 8 | 3 | 2 | 4 | 4 | NS |
| Alcohol intake | 12 | 3 | 1 | 1 | 1 | NS |
| PH of PUD | 8 | 0 | 6 | 7 | 0 | NS |
| FH of PUD | 11 | 3 | 5 | 6 | 3 | NS |
Basal plasma gastrin- 17 levels were compared between groups of patients at the beginning of the study. These findings are shown in Figure 1. PGL in H pylori+DU patients were significantly higher at the beginning of the study than in any other group of patients irrespective of the presence of infection (H pylori +DU: 106.78 ± 22.72 pg/mL vs H pylori +Ge: 74.95 ± 15.63, H pylori+Gne: 74.21 ± 10.99, CG1: 68.59 ± 17.97 and CG2: 39.24 ± 5.59 pg/mL, P < 0.001). Healthy asymptomatic controls (CG2) had significantly lower plasma gastrin levels then all other dyspeptic patients (P < 0.01). After 6 mo, statistically significant decrease of PGL were observed in all successfully eradicated patients (H pylori +DU: 59.93 ± 9.40 and H pylori+Ge: 42.36 ± 10.28 pg/mL, P < 0.001). However, no significant change was seen in other groups (H pylori+Gne: 76.81 ± 19.54 and CG1: 58.29 ± 17.97 pg/mL, P > 0.05).
At the beginning of the study there was no significant difference (P > 0.05) in antral tissue gastrin concentrations (ATGC) between groups of infected (H pylori+Ge: 0.23 ± 0.05, H pylori+Gne: 0.23 ± 0.05, H pylori+DU: 0.24 ± 0.03 pg/g wet weight) and uninfected patients (CG1: 0.22 ± 0.04 and CG2: 0.24 ± 0.03 pg/g wet weight). After successful eradication therapy significant decrease in ATGC was observed only in H pylori+Ge group (0.20 ± 0.05 pg/g wet weight, P < 0.05), while in H pylori+DU group no significant change was observed (0.22 ± 0.04 pg/wet weight, P > 0.05). ATGC in the H pylori+Gne and CG1 did not change significantly (0.26 ± 0.07 and 0.26 ± 0.06 pg/g wet weight, respectively, P > 0.05). These findings are seen in Figure 2.
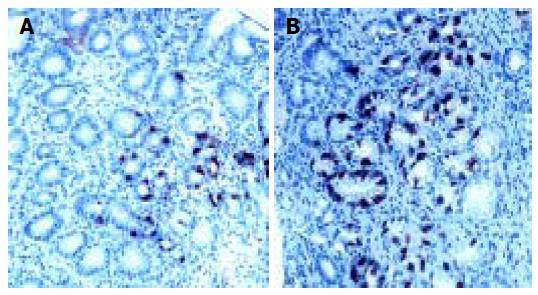
Antral G cell number was expressed as number of identified G cells per mm2 of antral mucosa, G cell volume density(%) and a ratio of antral G/total antral endocrine cell count (Table 2). Antral G cell number remained unchanged after 6 mo of follow-up in H pylori+Gne (405 ± 32 vs 432 ± 18), CG1 (413 ± 61 vs 428 ± 80) and CG2 (426 ± 57) patients. In the H pylori+DU patients mean number of antral G cells per mm2 of mucosa was lower at the beginning of the study (292 ± 20) than in any other group analyzed (P < 0.01). At the end of the follow-up and after successful eradication of H pylori infection number of antral G cells identified in DU patients was increased (400 ± 32) and comparable to both control groups (CG1 and CG2). By contrast, in patients with gastritis successful treatment lead to a decrease in both antral G cell number (416 ± 40 vs 294 ± 32, P < 0.01) and volume density (0.48 ± 0.09 vs 0.31 ± 0.02, P < 0.01), as seen in Figure 3. However, no significant change of the G cell/ total endocrine cell ratio was observed during the 6 mo of follow up in any of the groups, including H pylori +Ge group (Figure 4).
| H pylori +Ge | H pylori +Gne | H pylori +DU | CG1 | CG2 | P | |
| (n = 24) | (n = 7) | (n = 8) | (n = 11) | (n = 7) | ||
| G cell number (per mm2 of antral mucosa) | ||||||
| Before therapy | 416 ± 40 | 405 ± 32 | 292 ± 20a | 413 ± 61 | 426 ± 57 | 0.05a |
| After therapy | 294 ± 32a | 432 ± 18 | 400 ± 32b | 428 ± 80 | / | NS |
| P | 0.05a | NS | 0.01b | NS | / | |
| G cell volume density (%) | ||||||
| Before therapy | 0.48 ± 0.09 | 0.43 ± 0.11 | 0.42 ± 0.02 | 0.48 ± 0.03 | 0.44 ± 0.02 | NS |
| After therapy | 0.31 ± 0.02a | 0.40 ± 0.04 | 0.43 ± 0.01 | 0.50 ± 0.03 | / | NS |
| P | 0.05 | NS | NS | NS | / | |
| Antral G/ total antral endocrine cells ratio | ||||||
| Before therapy | 0.44 ± 0.04 | 0.40 ± 0.03 | 0.37 ± 0.05 | 0.44 ± 0.29 | 0.45 ± 0.07 | NS |
| After therapy | 0.44 ± 0.02 | 0.47 ± 0.07 | 0.41 ± 0.07 | 0.44 ± 0.37 | / | NS |
| P | NS | NS | NS | NS | / |
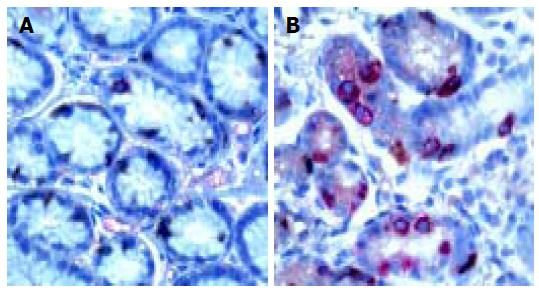
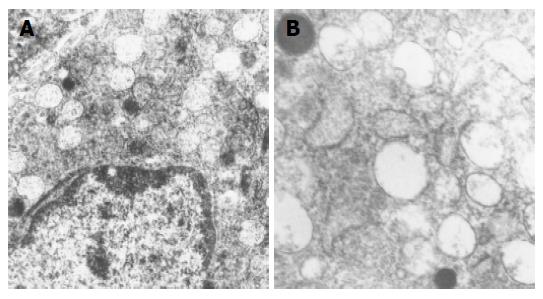
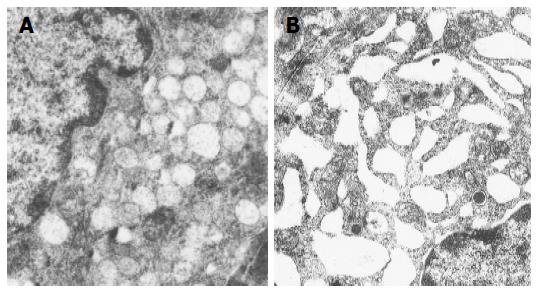
Ultrastructural characteristics of antral G cells in healthy controls (Figure 5) and dyspeptic patients (Figure 6) before and after therapy were assessed using electron microscopy morphometric study (Table 3). Electron microscopy revealed changes in antral G cell morphology in all H pylori+patients. Namely, more prominent endoplasmatic reticulum (Figure 6B) and Golgy apparatus together with an increase in number and volume density of cytoplasmic granules (Figure 6A) were identified in all H pylori infected individuals. Antral G cells of H pylori+DU patients, however, exhibited some specific features, such as increased cell profile surface and increased mean diameter of cytoplasmic granules. It was also found that G cells in DU patients, in comparison with healthy controls, have an increased proportion of dense core granules. In H pylori+Ge patients more granules per profile and higher volumen density, but similar diameter of secretory granules was identified when compared to the CG1 patients.
| Antral G cell | H pylori+Ge | H pylori+Gne | H pylori+DU | CG1 | CG2 | P |
| (n = 24) | (n = 7) | (n = 8) | (n = 11) | (n = 7) | ||
| Number of cells analyzed | ||||||
| Before therapy | 93 | 30 | 33 | 55 | 37 | |
| After therapy | 113 | 31 | 30 | 47 | ||
| Cell profile surface (μm2) | ||||||
| Before therapy | 142 ± 14 | 138 ± 20 | 188 ± 14a | 130 ± 10 | 136 ± 10 | 0.05a |
| After therapy | 140 ± 15 | 133 ± 12 | 138 ± 10d | 133 ± 12 | / | NS |
| P | NS | NS | 0.001d | NS | / | |
| Endoplasmatic reticulum (μm2) (profile surface) | ||||||
| Before therapy | 6.9 ± 2.3a | 6.2 ± 2.3 | 17.9 ± 2.3b | 5.3 ± 0.9 | 5.5 ± 0.9 | a0.05;b0.01 |
| After therapy | 5.0 ± 2.3 | 6.0 ± 1.0 | 5.7 ± 1.3d | 5.5 ± 0.7 | NS | |
| P | NS | NS | 0.001d | NS | ||
| Volumen density of cytoplasmic granules | ||||||
| Before therapy | 17.3 ± 1.9a | 13.1 ± 3.2 | 47.0 ± 4.9b | 14.6 ± 2.7 | 13.6 ± 2.7 | a0.05;b0.01 |
| After therapy | 9.7 ± 1.1b | 14.2 ± 3.1 | 17.3 ± 1.9 | 13.2 ± 2.0 | / | NS |
| P | 0.01b | NS | NS | NS | / | |
| Number of cytoplasmic granules/cell profile | ||||||
| Before therapy | 232 ± 18a | 199 ± 34 | 330 ± 13b | 190 ± 37 | 196 ± 21 | a0.05;b0.01 |
| After therapy | 180 ± 13 | 202 ± 45 | 220 ± 31 | 196 ± 20 | / | NS |
| P | NS | NS | NS | NS | / | |
| Mean diameter of cytoplasmic granules (nm) | ||||||
| Before therapy | 220 ± 23 | 245 ± 11 | 327 ± 23a | 237 ± 4 | 227 ± 7 | 0.01a |
| After therapy | 223 ± 23 | 229 ± 22 | 257 ± 21b | 223 ± 9 | / | NS |
| P | NS | NS | 0.01b | NS | / | |
| Golgi apparatus (μm2) (profile surface) | ||||||
| Before therapy | 2.9 ± 0.10b | 2.10 ± 0.3 | 3.33 ± 0.10a | 2.11 ± 0.33 | 2.05 ± 0.23 | 0.01a |
| After therapy | 2.15 ± 0.10 | 2.23 ± 0.2 | 2.20 ± 0.11b | 2.20 ± 0.22 | / | NS |
| P | 0.01b | NS | 0.01b | NS | / |
After successful eradication all those alterations were normalized and G cell ultrastructurally resembled to those found in CG2 group, with the exception of the diameter and volume density of cytoplasmic granules that remained higher in the H pylori +DU patients.
In our study, eradication therapy was successful in 82% (32/39) of all H pylori infected patients, all DU (100%, 8/8,) and 77% of patients (24/31) with gastritis that accords with previously proposed acceptable eradication rate of approximately 80%[2,22]. Eradication rate in our study is in line with results obtained with this eradication protocol that is, according to the literature, ranging from 53-81%[23,24].
It is well known that H pylori leads to chronic gastritis and affects gastric acid secretion in virtually all infected individuals. The underlying mechanism responsible for changes in gastric acid secretion in H pylori infected individuals is not completely explained, however it is probably a result of impaired gastrin and somatostatin secretion and elevated proinflammatory cytokines in the infected gastric mucosa[8,22,25]. Gastrin secretion and morphofunctional features of antral G cells in patients with H pylori infection and gastritis or DU was scarcely studied using a comparative study, before and after eradication of the infection.
In the present study, healthy asymptomatic individuals (CG2) had significantly lower plasma gastrin levels then all dyspeptic patients. Higher gastrin concentrations were registered in the plasma of H pylori infected individuals, both with gastritis and especially when duodenal ulcer was found on endoscopy. After 6 mo, significant decrease in plasma gastrin levels was registered in all successfully eradicated patients. However, no significant change was seen in other groups, including noneradicated patients with gastritis (H pylori +Gne) and dyspeptic patients without H pylori infection (CG1).
Higher basal plasma gastrin levels in H pylori infected patients and animals compared to the uninfected controls were previously reported by different authors[3-6,10,25]. Our results showed that in infected patients with DU have significantly higher plasma levels of gastrin -17 when compared to H pylori +patients with gastritis, as was previously demonstrated both in adults by Kamada et al[26], and in children by Kato et al[27], but opposed to the results of other studies[14]. After successful eradication of H pylori infection, plasma gastrin levels decreased markedly in both DU and gastritis patients, as demonstrated previously in adults[1,26,28-30] and pediatric patients[27,31] by other authors.
This change was not observed in non-eradicated patients and uninfected dyspeptic controls, implicating that decrease in plasma gastrin is directly related to the eradication of the bacterium from the stomach.
Antral tissue gastrin concentrations at the beginning of our study were similar in both infected (DU and gastritis group) and uninfected patients, as in other studies[31,32]. Successful eradication therapy lead to a significant decrease in antral tissue gastrin concentrations in patients with gastritis, as seen in other studies[33], but not in DU patients.
Immunohistochemical and ultrastructural examination of G cell morphology at the beginning of the study revealed significantly lower antral G cell number in H pylori+DU patients compared to all the other groups. However, after successful eradication antral G cell number was close to values observed in controls (both CG1 and CG2). By contrast, successful eradication therapy lead to a decrease in G cell number in the antrum of infected patients with gastritis. On the other hand no significant change in gastrin/total endocrine cell ratio was observed during the 6 mo of follow up, neither in infected nor uninfected patients.
Majority of authors agree that there is no significant difference in antral G cell number in infected and uninfected patients before therapy[12,26,28,31,32]. Yacoub et al in a similar study, did not find differences in antral G,D, and EC cell densities and G/D, G/EC and D/EC cell ratios in DU patients[13] compared to controls. On the other hand, a study by Chamouard et al[34], showed significantly lower number of antral G cells in DU patients, that is in agreement with the results of our study. The decrease in antral G cell number in subjects with DU (that can also correspond to the degranulation of very active gastrin-producing cells resulting in profound hypergastrinemia) could be related to the fact that the number of bacteria and/or severity of antral gastritis is greater in DU patients. Other possible explanation could be related to differences in H pylori strains that colonize the antrum of patients with DU. Namely, we previously reported that in Serbia and Montenegro there is high seroprevalence of cagA-positive H pylori strains in dyspeptic patients with and without peptic ulcer, while VacA-positive strains are more closely related to peptic ulcer disease[35]. However, Gaider et al found CagA+ strains in 78% patients with H pylori infection and DU and described the absence of CagA+strains in individuals with antral G-cell hyperplasia[36].
Based on the ultrastructural findings, we propose that in the H pylori +DU patients strongly stimulated remaining antral G cells, as a result of a compensatory mechanism, develop a strong synthetic and secretory phenotype before eradication therapy. In addition, after successful eradication all ultrastructural alterations registered in H pylori+DU patients were normalized to appearance expected in control group, excluding cytoplasmic granules volume density and their diameter which retained some higher values, but the observed difference was not statistically significant in comparison to values in healthy subjects. These findings suggest that remaining alterations in G cell morphology in DU should be attributed to an unknown host factor.
After successful H pylori eradication in dyspeptic patients with gastritis both unchanged[35] and decreased[31] antral G cell number was reported, that is consistent with our results where eradication therapy lead to a decrease in antral G cell number.
Ultrastructural examinations in H pylori+ patients with gastritis at the beginning of the study revealed similar, but less prominent subcellular alterations of G cell morphology detected the DU patients. We confirmed in our study, previously reported, increased cytoplasmic cell granule index in patients with H pylori-related gastritis using electron microscopy and immunohistochemical examination of antral G cells[12]. After successful eradication, all detected alterations were reversed and antral G cells had morphological features similar to those in healthy controls. This is consistent with the findings of Sugamata et al[14], demonstrating the reversibility of antral G cell morphology changes after elimination of the infection.
We identified total antral endocrine cell population and calculated number of G cells using double immunostaining for both synaptophysin and gastrin. Synaptophysin was considered an adequate pan-neuroendocrine marker based on the distribution background in the human antrum, while its importance in the other parts of gastrointestinal tract remains limited. The co-localization study revealed that synaptophysin immunoreactivity occurred in virtually all antral gastrin, somatostatin and serotonin-producing cells[38]. In the antrum, proportion G cells accounts for 40% of all endocrine cells[9], as described in all of our examined groups of patients and healthy controls. The fact that the antral G cells/total endocrine cell ratio remained unchanged after eradication of H pylori implicates that other gastric endocrine cell types disturbances are related with the presence of the infection. This finding is important for the understanding of the potential important role of other endocrine cell types and their interactive, functional relationship in gastric regulatory physiology in gastritis and peptic ulcer disease associated with H pylori infection. Namely, previous studies reported the increase in basal and gastrin- stimulated somatostatin-containing (D) cell activity in the early phase (4-8 wks) after H pylori eradication in gastric ulcers[22] and gastritis[39], suggesting that both antral G and D cell morphology and function should be considered in the gastric mucosal response to the presence of H pylori infection. Decreased antral D cell number in patients with H pylori-related chronic gastritis might be one of the reasons for the existing hypergastrinemia[12].
Possible mechanisms responsible for hypergastrinemia in H pylori associated gastritis or duodenal ulcer could be different. Some recent studies indicated that ypergastrinemia related to H pylori infection is associated with enhanced activity of platelet activating factor (PAF) produced locally, in the affected gastric mucosa and higher expression of nuclear factor kappaB (NF-kappaB)[40,41] . PAF may contribute to the hypergastrinemia of H pylori infection by stimulating gastrin release from G cells involving influx of extracellular calcium via L-type channels and activation of protein kinase C[40]. NF-kappaB activation is considered crucial event in the production of proinflammatory molecules in H pylori -associated gastritis. In the uninflammed stomach, NF-kappaB was highly expressed and active in a subset of epithelial cells, which were identified as predominantly G cells. In accordance with this activity, antral mucosa of infected individuals expressed high levels of the NF-kappaB target cytokine TNF-alpha, a well-documented stimulator of gastrin production. In patients with H pylori-associated gastritis, NF- kappaB activity was markedly enhanced and activation occurred preferentially in the epithelial cells[41]. In addition, treatment of cultured canine antral G-cells with H pylori constituents enhances subsequent basal and bombesin-stimulated gastrin release suggesting that direct contact between H pylori and G-cells in the gastric antrum may be responsible for the hypergastrinemia seen in the infected individuals[42].
In conclusion, it may be concluded that in H pylori infected patients with chronic gastritis, changes in morphology and function of antral G cell are completely reversible and attributable exclusively to the presence of the bacterium in the gastric mucosa. However, duodenal ulcer formation in infected individuals is partly attributable to the presence of the infection, but host factors are of importance as well, since more profound alterations are observed and a certain extent of antral G cell hyperfunction, resulting in prolonged hypergastrinemia, is detected. Further investigations are needed in order to identify relevant host characteristics leading to the ulcer formation including gene polymorphisms of proinflammatory cytokines involved in the control of gastric acid secretion.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Bobrzyński A. Hormonal, secretory and morphological alterations in gastric mucosa in the course of Helicobacter pylori eradication in patients with duodenal ulcer and non-ulcer dyspepsia. J Physiol Pharmacol. 1997;48 Suppl 3:1-56. [PubMed] |
| 2. | Hunt RH, Huang JQ. The case for treatment of dyspeptic patients infected with H. pylori. Eur J Surg Suppl. 1998;582:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Dockray GJ. Topical review. Gastrin and gastric epithelial physiology. J Physiol. 1999;518:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Kaneko H, Konagaya T, Kusugami K. Helicobacter pylori and gut hormones. J Gastroenterol. 2002;37:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Gisbert JP, Boixeda D, Vila T, de Rafael L, Redondo C, de Argila CM. Basal and stimulated gastrin levels and gastric acid output five months after therapy for Helicobacter pylori eradication in duodenal ulcer patients. J Clin Gastroenterol. 1996;22:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Haruma K, Sumii K, Okamoto S, Yoshihara M, Sumii M, Kajiyama G, Wagner S. Helicobacter pylori infection is associated with low antral somatostatin content in young adults. Implications for the pathogenesis of hypergastrinemia. Scand J Gastroenterol. 1995;30:550-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Feldman M, Cryer B, Lee E. Effects of Helicobacter pylori gastritis on gastric secretion in healthy human beings. Am J Physiol. 1998;274:G1011-G1017. [PubMed] |
| 8. | Hiraoka S, Miyazaki Y, Kitamura S, Toyota M, Kiyohara T, Shinomura Y, Mukaida N, Matsuzawa Y. Gastrin induces CXC chemokine expression in gastric epithelial cells through activation of NF-kappaB. Am J Physiol Gastrointest Liver Physiol. 2001;281:G735-G742. [PubMed] |
| 9. | Sugiyama A, Ikeno T, Maruta F, Kawasaki S, Hayama M, Ota H, Yoshizawa A, Nakajima K, Fukushima M, Honda T. Long-term Helicobacter pylori colonization produces G cell hyperplasia and carcinoid tumor in Mongolian gerbils. J Cell Mol Med. 2000;4:308-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Peek RM, Wirth HP, Moss SF, Yang M, Abdalla AM, Tham KT, Zhang T, Tang LH, Modlin IM, Blaser MJ. Helicobacter pylori alters gastric epithelial cell cycle events and gastrin secretion in Mongolian gerbils. Gastroenterology. 2000;118:48-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 138] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Drndarevic N, Todorovic V, Micev M, Sokic-Milutinovic A, Milosavljevic T, Spuran M. Assesment of epithelial cell prolif-eration in gastric mucosal biopsies of patients with Helicobacter pylori infection. Gut. 2003;52:A151. |
| 12. | Tzaneva M. Light and electron microscopic immunohistochemical investigation on G and D cells in antral mucosa in Helicobacter pylori-related gastritis. Exp Toxicol Pathol. 2001;52:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Yacoub WR, Thomson AB, Hooper P, Jewell LD. Immunocytochemical and morphometric studies of gastrin-, somatostatin- and serotonin-producing cells in the stomach and duodenum of patients with acid peptic disorders. Can J Gastroenterol. 1996;10:395-400. [PubMed] |
| 14. | Sugamata M, Ihara T, Todate A, Sugamata M, Hirakawa R, Yoshida Y, Yamanaka T, Miyata M, Miura M. Ultrastructural study of antral G cells in patients with duodenal ulcer: effect of Helicobacter pylori eradication. Helicobacter. 1997;2:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 15. | Malfertheiner P, Mégraud F, O'Morain C, Bell D, Bianchi Porro G, Deltenre M, Forman D, Gasbarrini G, Jaup B, Misiewicz JJ. Current European concepts in the management of Helicobacter pylori infection--the Maastricht Consensus Report. The European Helicobacter Pylori Study Group (EHPSG). Eur J Gastroenterol Hepatol. 1997;9:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 160] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Goodwin CS. The Sydney System: microbial gastritis. J Gastroenterol Hepatol. 1991;6:235-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3535] [Article Influence: 121.9] [Reference Citation Analysis (3)] |
| 18. | Sternberg LA. Immunohistochemistry. New York: Zwiley and Sons 1986; . |
| 19. | Koko V, Todorović V, Varagić J, Micev M, Korać A, Bajcetić M, Cakić-Milosević M, Nedeljković M, Drndarević N. Gastrin producing G-cells after chronic ethanol and low protein nutrition. Indian J Exp Biol. 1998;36:1093-1101. [PubMed] |
| 20. | Todorović V, Koko V, Varagić J, Lacković V, Vuzevski V, Milin J. Effects of chronic ethanol administration on the serotonin-producing cells in rat gastric antral and duodenal mucosa. Histol Histopathol. 1993;8:285-296. [PubMed] |
| 21. | Bordi C, D'Adda T, Azzoni C, Ferraro G. Classification of gastric endocrine cells at the light and electron microscopical levels. Microsc Res Tech. 2000;48:258-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Hayakawa T, Kaneko H, Konagaya T, Shinozaki K, Kasahara A, Funaki Y, Mori S, Yokoi T, Hirooka Y, Kusugami K. Enhanced somatostatin secretion into the gastric juice with recovery of basal acid output after Helicobacter pylori eradication in gastric ulcers. J Gastroenterol Hepatol. 2003;18:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Lind T, Veldhuyzen van Zanten S, Unge P, Spiller R, Bayerdörffer E, O'Morain C, Bardhan KD, Bradette M, Chiba N, Wrangstadh M. Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: the MACH I Study. Helicobacter. 1996;1:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 412] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 24. | Bayerdörffer E, Lind T, Díte P, Bardhan KD, O'Morain C, Delchier JC, Spiller R, Veldhuyzen van Zanten S, Sipponen P, Mégraud F. Omeprazole, amoxycillin and metronidazole for the cure of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1999;11 Suppl 2:S19-22; discussion S23-4. [PubMed] |
| 25. | Zhao CM, Wang X, Friis-Hansen L, Waldum HL, Halgunset J, Wadström T, Chen D. Chronic Helicobacter pylori infection results in gastric hypoacidity and hypergastrinemia in wild-type mice but vagally induced hypersecretion in gastrin-deficient mice. Regul Pept. 2003;115:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Kamada T, Haruma K, Kawaguchi H, Yoshihara M, Sumii K, Kajiyama G. The association between antral G and D cells and mucosal inflammation, atrophy, and Helicobacter pylori infection in subjects with normal mucosa, chronic gastritis, and duodenal ulcer. Am J Gastroenterol. 1998;93:748-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Kato S, Ozawa K, Koike T, Sekine H, Ohara S, Minoura T, Iinuma K. Effect of Helicobacter pylori infection on gastric acid secretion and meal-stimulated serum gastrin in children. Helicobacter. 2004;9:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Park SM, Lee HR, Kim JG, Park JW, Jung G, Han SH, Cho JH, Kim MK. Effect of Helicobacter pylori infection on antral gastrin and somatostatin cells and on serum gastrin concentrations. Korean J Intern Med. 1999;14:15-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Kim JH, Park HJ, Cho JS, Lee KS, Lee SI, Park IS, Kim CK. Relationship of CagA to serum gastrin concentrations and antral G, D cell densities in Helicobacter pylori infection. Yonsei Med J. 1999;40:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Williams MP, Usselmann B, Chilton A, Sercombe J, Nwokolo CU, Pounder RE. Eradication of Helicobacter pylori increases nocturnal intragastric acidity during dosing with rabeprazole, omeprazole, lansoprazole and placebo. Aliment Pharmacol Ther. 2003;17:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Queiroz DM, Mendes EN, Rocha GA, Moura SB, Resende LM, Barbosa AJ, Coelho LG, Passos MC, Castro LP, Oliveira CA. Effect of Helicobacter pylori eradication on antral gastrin- and somatostatin-immunoreactive cell density and gastrin and somatostatin concentrations. Scand J Gastroenterol. 1993;28:858-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Odum L, Petersen HD, Andersen IB, Hansen BF, Rehfeld JF. Gastrin and somatostatin in Helicobacter pylori infected antral mucosa. Gut. 1994;35:615-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Kwan CP, Tytgat GN. Antral G-cell hyperplasia: a vanishing disease? Eur J Gastroenterol Hepatol. 1995;7:1099-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Chamouard P, Walter P, Wittersheim C, Demuynck P, Meunier O, Baumann R. Antral and fundic D-cell numbers in Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1997;9:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Sokic-Milutinovic A, Wex T, Todorovic V, Milosavljevic T, Malfertheiner P. Anti-CagA and anti-VacA antibodies in Helicobacter pylori-infected patients with and without peptic ulcer disease in Serbia and Montenegro. Scand J Gastroenterol. 2004;39:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Gaidar IuA, Stepanova EV, Mosiichuk LN, Demeshkina LV, Sheikhetova GA, Bespalova EV, Kudriavtseva VE, Vcherashniaia NN. [Particular features of the relationship of Helicobacter pylori and G cells in patients with duodenal ulcer, with special reference to the Helicobacter pylori strain]. Lik Sprava. 2001;4:176-177. [PubMed] |
| 37. | Graham DY, Lew GM, Lechago J. Antral G-cell and D-cell numbers in Helicobacter pylori infection: effect of H. pylori eradication. Gastroenterology. 1993;104:1655-1660. [PubMed] |
| 38. | Portela-Gomes GM, Stridsberg M, Johansson H, Grimelius L. Co-localization of synaptophysin with different neuroendocrine hormones in the human gastrointestinal tract. Histochem Cell Biol. 1999;111:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Milutinovic AS, Todorovic V, Milosavljevic T, Micev M, Spuran M, Drndarevic N. Somatostatin and D cells in patients with gastritis in the course of Helicobacter pylori eradication: a six-month, follow-up study. Eur J Gastroenterol Hepatol. 2003;15:755-766. [PubMed] |
| 40. | Beales IL. Effect of platelet-activating factor on gastrin release from cultured rabbit G-cells. Dig Dis Sci. 2001;46:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | van Den Brink GR, ten Kate FJ, Ponsioen CY, Rive MM, Tytgat GN, van Deventer SJ, Peppelenbosch MP. Expression and activation of NF-kappa B in the antrum of the human stomach. J Immunol. 2000;164:3353-3359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Beales IL, Calam J. Helicobacter pylori increases gastrin release from cultured canine antral G-cells. Eur J Gastroenterol Hepatol. 2000;12:641-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |