Published online Jul 7, 2005. doi: 10.3748/wjg.v11.i25.3920
Revised: November 20, 2004
Accepted: November 24, 2004
Published online: July 7, 2005
AIM: To investigate the protective effect of isoflurane on energy balance in isolated hepatocytes during in vitro anoxia/reoxygenation, and to compare isoflurane with halothane.
METHODS: Hepatocytes freshly isolated from fed rats were suspended in Krebs-Henseleit buffer, and incubated in sealed flasks under O2/CO2 or N2/CO2 (95%/5%, V/V) for 30 or 60 min, followed by 5 or 10 min of reoxygenation, with an added volatile anesthetic or not. ATP, ADP, and adenosine monophosphate in hepatocytes were determined by high performance liquid chromatography, and energy charge was calculated.
RESULTS: During 30 min of anoxia, the energy charge and total adenine nucleotide steadily increased with the isoflurane dose from 0 to 2 minimum alveolar anesthetic concentration (MAC), then decreased from 2 to 3 MAC. In short incubations (30-35 min) at 1 MAC isoflurane, energy charge modestly decreased during anoxia, which was partially prevented by isoflurane and completely reversed by reoxygenation, and total adenine nucleotide did not decrease. In long incubations (60-70 min), both energy charge and total adenine nucleotide greatly decreased during anoxia, with partial and no reversal by reoxygenation, respectively. Isoflurane partly prevented decreases in both energy charge and total adenine nucleotide during anoxia and reoxygenation. In addition, 1 MAC isoflurane obviously increased ATP/ADP, which could not be changed by 1 MAC halothane.
CONCLUSION: Isoflurane partially protects isolated hepatocytes against decreases in both energy charge and total adenine nucleotide during short (reversible) or long (irreversible) anoxia.
-
Citation: Li Q, Yu WF, Zhou MT, Lu X, Yang LQ, Zhu M, Song JG, Lu JH. Isoflurane preserves energy balance in isolated hepatocytes during
in vitro anoxia/reoxygenation. World J Gastroenterol 2005; 11(25): 3920-3924 - URL: https://www.wjgnet.com/1007-9327/full/v11/i25/3920.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i25.3920
Hepatic anoxia, alone or as a component of ischemia, is an ever-present concern during abdominal surgery, because associated inhibition of energy supply threatens liver cell function and viability[1,2]. Evidence is mounting that the inability of the liver to maintain or regain energy balance during and after surgery is one of the strongest predictors of liver damage and adverse outcome[3,4]. Also, release from injured tissue of metabolites, such as adenosine, with cardiovascular effects may further compromise the anesthetic management of seriously ill or injured patients. Thus, surgeons and anesthesiologists need to be aware of, and to use, whatever measures are available to preserve energy balance in tissues.
The sum of ATP splitting by many concurrent energy-requiring reactions is called “ATP demand.” ATP supply occurs mainly via mitochondrial oxidative phosphorylation, which is absolutely dependent on O2. Under normal conditions, ATP supply easily keeps pace with ATP demand, and adenine nucleotide (high-energy phosphate) exists mainly in the form of ATP, along with relatively small amounts of ADP and adenosine monophosphate (AMP). However, when ATP supply is inhibited by lack of oxygen, ATP demand predo-minates, ADP and AMP then accumulate at the expense of ATP, and eventually adenosine and other non-nucleotide metabolites appear. Thus, shifts in the balance between ATP supply and demand can be assessed by measuring changes in the absolute and relative levels of ATP and its metabolites. A more complete and accurate expression is energy charge. Energy charge = (ATP+1/2ADP)/(ATP+ADP+AMP).
Hepatocytes were isolated from adult male Sprague-Dawley rats (250-300 g) having free access to food and water[5]. Livers were perfused in situ by using Ca2+-free Krebs-Henseleit buffer (pH 7.4) supplemented with 20 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, maintained at 37°C and equilibrated with O2/CO2 (95/5). Perfusion was continued for 10 min with buffer alone, then for another 12-14 min with added collagenase (Type I, Sigma Chemical Co., St. Louis, MO). The softened liver was transferred to a plastic weighing dish containing 25 mL Krebs+2% dissolved bovine serum albumin, teased apart with a spatula and chopped finely with sharp scissors. After further dilution to 100 mL with Krebs+2% dissolved bovine serum albumin, the cell slurry was washed into a 500-mL Erlenmeyer flask, gently swirled under a flowing O2/CO2 (95%/5% V/V) atmosphere at 37°C for 15 min, then filtered through nylon mesh. Each 12 mL of crude cell suspension was mixed with 28 mL Percoll (Pharmacia, Sweden, obtained from Sigma) and centrifuged at 10 000 g for 10 min. The layer of intact, purified hepatocytes at the bottom of the gradient was rinsed free of Percoll by suspension in Krebs and centrifugation for 2 min at 50 r/min. The final pellet contained a total of 2-4 × 108 cells that were 90-95% viable by dye exclusion. Cells were stored for 2 h on ice before use without loss of viability.
In 25-mL round-bottomed flasks, 12.5 million cells were suspended in a total volume of 2.5 mL Krebs+2% dissolved bovine serum albumin (pH 7.4). Flasks were sealed with rubber caps through which 14-gauge needles were inserted for in- and out-flow of gas mixture. After 10 min preincubation under O2/CO2, regassing and experimental incubations were carried out as follows: O2/CO2 for 35 or 70 min (= oxygenated), N2/CO2 for 30 or 60 min (= anoxic), or N2/CO2 for 30 or 60 min followed by O2/CO2 for 5 or 10 min, respectively (= reoxygenated). All incubations were performed by swirling the flasks in a water bath at 37°C. When needed, anesthetics were added at the desired concentrations to the gas mixture used for gassing the flasks by means of a copper kettle vaporizer. Gas chromatography measurements established that anesthetic concentrations in liquid phase reached a constant value within 5-10 min. (The absolute concentrations in the liquid phase varied with anesthetic dose and cell concentration.) Incubations were terminated by injecting 0.5 mL 2 mol/L perchloric acid forcefully into the suspension to arrest enzyme-catalyzed reactions. After removal of preci-pitated membranes and protein by centrifugation, the clear supernatant containing extracted adenine nucleotides and other metabolites was neutralized with 2 mol/L potassium hydroxide and cooled on ice to precipitate excess potassium perchlorate. The supernatant was decanted and stored at-20°C before metabolite measurements.
ATP, ADP, and AMP were analyzed by high performance liquid chromatography (LDC Analytical, Riviera Beach, FL, USA) using a CM4000 pump interfaced with a SM5000 detector. The separation was accomplished on a C18 reversed phase column. Elution with a binary gradient was carried out at a flow rate of 1.0 mL/min. Mobile phase A consisted of 30 mmol/L potassium phosphate as buffer (pH 6.0) and 8 mmol/L tetrabutylammonium hydrogen sulfate as ion-pairing reagent. Mobile phase B was identical except that it contained 500 mL/L methanol. Recovery of ATP, ADP, and AMP routinely exceeded 90-95%, as estimated from the concentration of caffeine added as an internal standard.
Data were presented as mean±SD. One- or two-way analysis of variance with replications and Scheffe’s or paired t-tests were used for statistical analysis. P < 0.05 was considered statistically significant.
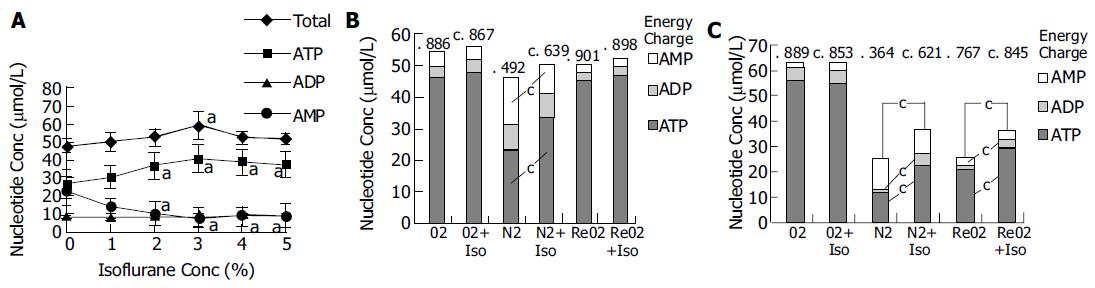
Figure 1A shows the effect of isoflurane dose on adenine nucleotide levels in isolated hepatocytes after exposure to anoxia for 30 min. ATP levels increased and AMP levels decreased from their respective control values as isoflurane increased from 0 to 2 minimum alveolar anesthetic concentration (MAC, 0-3% concentration), with a slight reversal from 2 to 3 MAC (3-5% concentration). Total adenine nucleotide increased to a lesser degree with isoflurane dose and was significantly higher than baseline only at 2 MAC. Values of ADP did not change significantly from baseline at any dose of isoflurane. Values of energy charge (not shown) paralleled to those of ATP.
Figure 1B shows data obtained from incubations performed for 30-35 min. In cells incubated under O2 for 35 min, amounts of ATP were maximal and balanced by relatively small amounts of ADP and AMP. Isoflurane slightly decreased ATP while increasing ADP and AMP. Although changes in individual nucleotide concentrations were not statistically significant, they combined to produce a significant decrease in energy charge.
In cells anoxic for 30 min (under N2 rather than O2), ATP substantially decreased and ADP and AMP increased compared to oxygenated cells. The associated decrease in energy charge confirmed that anoxia shifted energy balance toward a much lower degree of phosphorylation in the adenine nucleotide pool. When isoflurane was present during anoxia, ATP remained significantly higher and AMP lower than in anoxic cells without isoflurane. These two effects of isoflurane combined to maintain a proportionately higher value of energy charge.
Cells exposed to 30 min of anoxia followed by 5 min of reoxygenation showed values of all three adenine nucleotides and energy charge that were not significantly different from those of cells exposed to O2 continuously for 30 min. Furthermore, no difference in any of these variables was seen for +isoflurane compared to -isoflurane.
Figure 1C shows results obtained from a second set of cell preparations subjected to incubations for 60-70 min. In cells incubated under O2 alone for 70 min, absolute and relative amounts of ATP, ADP, and AMP were not significantly different from those incubated for 30 min. The changes produced by inclusion of isoflurane along with O2 decreased ATP, increased ADP and AMP, and a statis-tically significant decrease in energy charge were only slightly (and not significantly) larger than in cells incubated for 35 min.
In cells that were anoxic (exposed to N2) for 60 min, values of ATP, ADP, AMP, total adenine nucleotide, and energy charge were all significantly lower than in cells exposed anoxia for 30 min. The effects of including isoflurane during anoxia were also generally more pronounced in cells exposed to anoxia for 60 min: compared to values in the absence of isoflurane, ATP was more than double and energy charge almost double, ADP and total adenine nucleotide were significantly higher while AMP was not significantly lower.
Reoxygenated cells incubated for longer periods (N2 for 60 min, O2 for 10 min) differed greatly from cells subjected to shorter incubations (N2 30 min, O2 5 min), whereas values of ADP and AMP were not much different from those of oxygenated cells. Energy charge, ATP and total adenine nucleotide were drastically decreased. Another difference between longer and shorter incubations of reoxygenated cells was that in the longer ones, isoflurane-related differences in energy status persisted into the reoxygenation period; increases in ATP, ADP, and total adenine nucleotide during anoxia were maintained during reoxygenation.
Table 1 shows the data from a separate experiment carried out solely to compare isoflurane and halothane at approximately 1 MAC for their ability to alter energy status in isolated hepatocytes during 30-min exposure to anoxia. ATP/ADP was again higher with 1 MAC isoflurane present than in paired control incubation (isoflurane absent) using cells from the same preparation. With 1 MAC halothane, no difference at all in ATP/ADP during anoxia was seen, each incubated in the presence and absence of that agent.
Intact isolated hepatocytes embody a physiologic balance of the reactions involved in ATP supply and demand in a form that permits uniform control and ready measurement of biochemical variables. We have studied these cells extensively under simulated intra-operative conditions to predict energetic responses at the tissue and organ level.
The data in Figure 1B show that the energy-protective effect of isoflurane in anoxic hepatocytes varied in a systematic way with isoflurane dose. There was a graded dose-response relationship from 0 to 2 MAC, with a slight reversal of protection at 3 MAC. It seems that, at least in isolated cells, the energy-protective effect of isoflurane is optimal in the dose range used for clinical anesthesia. The basis for the response “ceiling” at 2 MAC awaits an understanding of the biochemical basis of the protective effect. An important question still to be addressed in this line of investigation is the extent to which the protective effect is associated with the anesthetic state. Is this effect limited to isoflurane or to other halogenated or volatile agents? Does ED50 for the protective effect correlate with MAC?
In the shorter incubation (30-35 min) that examined varying oxygenation status, 1 MAC isoflurane tended to decrease ATP and increase ADP and AMP in cells exposed to oxygen, presumably because isoflurane inhibits mitochondrial recycling of ADP to ATP via oxidative phosphorylation[6,7]. An opposite and larger effect of isoflurane on energy status was found in anoxic cells: superimposed on the great decrease in ATP and energy charge produced by anoxia, the effect of isoflurane was to enhance energy balance rather than to further impair it. In reoxygenated cells, there were neither protective nor detrimental effects of isoflurane on energy balance; and isoflurane produced no effect at all on any of the measured variables of energy status. The similarity between reoxygenated and continuously oxygenated cells indicates that exposure to 30-min anoxia with or without isoflurane has no irreversible effect on the energy status of hepatocytes.
The longer incubation (60-70 min) produced physiologically significant and/or irreversible effects of anoxia on cellular energy status. As in the 35-min incubation, data from cells oxygenated for 70 min showed that non-anoxic injury might have occurred during that period of incubation. The similarities in ATP, ADP, AMP, and total adenine nucleotide values suggest that the longer period of incubation is well tolerated, because damaged cells rapidly lose adenine nucleotides. The effects of anoxia on adenine nucleotide content and energy balance were considerably greater after 60-min exposure to anoxia than after 30-min exposure to anoxia. In anoxic cells after 60-min exposure to anoxia, there was a great decrease in total adenine nucleotide compared to oxygenated cells (vs none at 30 min), the decrease in energy charge was also greater after 60-min exposure to anoxia than after 30-min exposure to anoxia. In reoxygenated cells, none of the effects of 60-min exposure to anoxia were reversed, in contrast to all of the effects of 30-min exposure to anoxia. The importance of total adenine nucleotide in limiting the levels of ATP, ADP, and AMP during brief recovery from anoxia was shown clearly by the almost identically lowered values of total adenine nucleotide in corresponding anoxic and reoxygenated cells after 70-min exposure to anoxia. Also, in contrast to the shorter incubation, in the longer incubation the protective effect of isoflurane was observed during anoxia-increased energy charge and total adenine nucleotide was persistent through the reoxygenation period.
As mentioned earlier, the measured values of ATP, ADP, and AMP concentrations and the calculated values of total adenine nucleotide and energy charge in isolated hepatocytes are sensitive indicators of changes in the dynamic balance of ATP supply and demand. The anesthetic effects reported here may well be due to the direct action of the drug on one or more of the intracellular reactions involved in producing or consuming ATP[8-10]. The results of other studies suggested that the protective effect of isoflurane may be due to “decreased metabolism” and, more precisely, due to inhibition of ATP demand[11-13]. The conclusion holds also for the findings of the present study. Previous studies showed that isoflurane causes a dose-related decrease in O2 consumption in isolated hepatocytes[14]. However, it would be erroneous to conclude that this decrease in O2 consumption reflects primary inhibition of ATP demand (with secondary inhibition of mitochondrial oxidative phosphorylation via respiratory control), because volatile anesthetics are also able to inhibit oxidative phosphorylation directly[15,16]. In fact, inhibition of ATP demand in intact cells can be ascertained only by direct measurement of ATP consumption.
Two possible mechanisms may be mentioned by which decreased AMP formation can maintain ATP levels. An anoxia-induced increase in AMP promotes its degradation to adenosine and other non-nucleotide metabolites[17]. The latter step seems to be a physiologic “point of no return”, because ATP is not easily resynthesized from its non-nucleotide metabolites when oxygen and ATP supply are restored[17]. Thus, a decrease in AMP formation would decrease the rate of ensuing AMP degradation and loss of total adenine nucleotide. Also, a decrease in AMP formation from ADP via inhibition of adenylate kinase can preserve the availability of ADP for conversion back to ATP via glycolysis.
The present study demonstrates that 1 MAC isoflurane actually helps to preserve liver cell energy balance during anoxia (whereas 1 MAC halothane has no such effect). The demonstration of this protective effect might be crucially dependent on the specific experimental conditions used in this study. Relatively short periods of anoxic exposure may prevent enzymes that catalyze ATP-consuming reactions from being degraded to the extent that inhibition of their activity by isoflurane could not be detected. Also, the use of anoxia (0% O2) rather than hypoxia may eliminate vestiges of mitochondrial ATP formation, anesthetic inhibition of which may decrease ATP/ADP and thus opposing or even canceling ATP/ADP increases resulting from anesthetic inhibition of ATP consumption[18,19].
For optimal anesthetic and surgical care, we need to know as much as possible about the effects and actions of the drugs we use, especially with regard to a process as essential as energy balance. The work described here has documented certain effects of isoflurane on ATP supply and demand at the cellular level and laid the groundwork for explaining them in terms of actions on specific biochemical reactions that produce and consume ATP. In addition to further elucidate such basic mechanisms, we also need to examine the consequence of these cellular effects in physiologic and clinical terms, by using more intact systems and measures of outcome.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Vagts DA, Iber T, Puccini M, Szabo B, Haberstroh J, Villinger F, Geiger K, Nöldge-Schomburg GF. The effects of thoracic epidural anesthesia on hepatic perfusion and oxygenation in healthy pigs during general anesthesia and surgical stress. Anesth Analg. 2003;97:1824-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Ishida H, Kadota Y, Sameshima T, Nishiyama A, Oda T, Kanmura Y. Comparison between sevoflurane and isoflurane anesthesia in pig hepatic ischemia-reperfusion injury. J Anesth. 2002;16:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Net M, Valero R, Almenara R, Deulofeu R, López-Boado MA, Capdevila L, Barros P, Bombí JA, Agustí M, Adalia R. Hepatic preconditioning after prolonged warm ischemia by means of S-adenosyl-L-methionine administration in pig liver transplantation from non-heart-beating donors. Transplantation. 2003;75:1970-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Diskin MG, Mackey DR, Roche JF, Sreenan JM. Effects of nutrition and metabolic status on circulating hormones and ovarian follicle development in cattle. Anim Reprod Sci. 2003;78:345-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 228] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Liu XL, Li LJ, Chen Z. Isolation and primary culture of rat hepatocytes. Hepatobiliary Pancreat Dis Int. 2002;1:77-79. [PubMed] |
| 6. | da-Silva WS, Gómez-Puyou A, de Gómez-Puyou MT, Moreno-Sanchez R, De Felice FG, de Meis L, Oliveira MF, Galina A. Mitochondrial bound hexokinase activity as a preventive antioxidant defense: steady-state ADP formation as a regulatory mechanism of membrane potential and reactive oxygen species generation in mitochondria. J Biol Chem. 2004;279:39846-39855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 234] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | Ason B, Handayani R, Williams CR, Bertram JG, Hingorani MM, O'Donnell M, Goodman MF, Bloom LB. Mechanism of loading the Escherichia coli DNA polymerase III beta sliding clamp on DNA. Bona fide primer/templates preferentially trigger the gamma complex to hydrolyze ATP and load the clamp. J Biol Chem. 2003;278:10033-10040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Leal NA, Olteanu H, Banerjee R, Bobik TA. Human ATP: Cob(I)alamin adenosyltransferase and its interaction with methionine synthase reductase. J Biol Chem. 2004;279:47536-47542. [PubMed] |
| 9. | Halestrap AP. Mitochondrial permeability: dual role for the ADP/ATP translocator? Nature. 2004;430:1 p following 983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Kokoszka JE, Waymire KG, Levy SE, Sligh JE, Cai J, Jones DP, MacGregor GR, Wallace DC. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 815] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 11. | Korzeniewski B. Influence of substrate activation (hydrolysis of ATP by first steps of glycolysis and beta-oxidation) on the effect of enzyme deficiencies, inhibitors, substrate shortage and energy demand on oxidative phosphorylation. Biophys Chem. 2003;104:107-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Koebmann BJ, Westerhoff HV, Snoep JL, Solem C, Pedersen MB, Nilsson D, Michelsen O, Jensen PR. The extent to which ATP demand controls the glycolytic flux depends strongly on the organism and conditions for growth. Mol Biol Rep. 2002;29:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Korzeniewski B. Parallel activation in the ATP supply-demand system lessens the impact of inborn enzyme deficiencies, inhibitors, poisons or substrate shortage on oxidative phosphorylation in vivo. Biophys Chem. 2002;96:21-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Pathak BL, Becker GL, Reilly PJ, Hanson KA, Landers DF. Isoflurane partially preserves energy balance in isolated hepatocytes during in vitro anoxia. Anesth Analg. 1991;72:571-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Kayser EB, Morgan PG, Sedensky MM. Mitochondrial complex I function affects halothane sensitivity in Caenorhabditis elegans. Anesthesiology. 2004;101:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Liu ZH, He Y, Jin WQ, Chen XJ, Shen QX, Chi ZQ. Effect of chronic treatment of ohmefentanyl stereoisomers on cyclic AMP formation in Sf9 insect cells expressing human mu-opioid receptors. Life Sci. 2004;74:3001-3008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Vincent MF, Van den Berghe G, Hers HG. The pathway of adenine nucleotide catabolism and its control in isolated rat hepatocytes subjected to anoxia. Biochem J. 1982;202:117-123. [PubMed] |
| 18. | Becker GL, Hensel P, Holland AD, Miletich DJ, Albrecht RF. Energy deficits in hepatocytes isolated from phenobarbital-treated or fasted rats and briefly exposed to halothane and hypoxia in vitro. Anesthesiology. 1986;65:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Kenniston JA, Baker TA, Fernandez JM, Sauer RT. Linkage between ATP consumption and mechanical unfolding during the protein processing reactions of an AAA+ degradation machine. Cell. 2003;114:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 236] [Article Influence: 10.7] [Reference Citation Analysis (0)] |