INTRODUCTION
Budd-Chiari syndrome (BCS) is defined as obstructed hepatic venous outflow due to occlusion of the hepatic veins or inferior vena cava. In western societies, BCS is caused mainly by thrombosis of the hepatic veins[1]. The etiology of BCS caused by hepatic vein thrombosis can be diverse. The hematological diseases associated with BCS are polycythemia vera and paroxysmal nocturnal hemoglobinuria[2,3]. Other acquired risk factors for BCS include antiphospholipid syndrome, abdominal trauma, the use of oral contraceptives[4], pregnancy[1], and so on. In this case report, we describe a young female with BCS who did not have these risk factors, and was suffering from a superior vena cava occlusion.
CASE REPORT
A 31-year-old Japanese woman was admitted to hospital because of a tender and distended abdomen, which had started 3 mo before. The patient had been in excellent health. The patient had taken no oral contraceptives. Both her mother and father were Japanese. The family history of venous thrombo-embolism was negative for third-degree relatives. A systemic examination revealed that the patient was 163.2 cm tall, 57.5 kg in weight, had a heart rate of 60/min, respiratory rate of 24/min, and blood pressure of 115/60 mmHg. A distended abdomen, with dilated veins on the anterior and posterior aspects of both the chest and abdominal wall were recognized. Routine laboratory tests were then performed. Liver function tests were almost normal, with albumin and total protein levels of 3.3 g/dL (normal range 4.0-5.0 g/dL) and 5.3 g/dL (normal range 6.7-8.3 g/dL), respectively. The serum electrolytes and renal function tests were within normal limits. Coagulation tests were almost normal, with a fibrinogen and fibrin degradation products level of 46.5 g/mL (normal range below 10 g/mL), and a α2 plasmin inhibitor-plasmin complex level of 2.8 g/mL (normal range below 0.8 g/mL). Prothrombin time, activated partial thromboplastin time, the levels of D-dimer, antithrombin-III, and both protein S and C were normal. The ascites was aspirated and analyzed. Ascites consisted of a transudate, and neither malignant cells nor bacteria were recognized. There was no evidence for autoimmune diseases or anticardiolipin antibody syndrome with the normal antinuclear antibody, anticardiolipin antibody, and lupus anticoagulant levels. In addition, there was no evidence for paroxysmal nocturnal hemoglobinuria (acid Ham test and an immunofluorescence test on granulocytes for the expression of phosphatidylinositol proteins). An abdominal ultrasound revealed an enlarged liver with impeded venous outflow and massive ascites. On computer tomographic examination, the liver was enlarged and a heterogeneous distribution of enhanced medium was observed. The hepatic vein was not recognized, which suggested BCS (Figure 1). On cavography via the femoral vein, normal hepatic vein was not visualized. Extensive collateral circulation, which was believed to be the right accessory hepatic vein, was visualized and flowed into the inferior vena cava. The orifice of this collateral vein was remarkably stenotic, but there were no signs of membranous obstruction of the inferior vena cava (Figures 2A and 2B). On cavography via bilateral median basilic veins, complete obstruction from the axillary to the subclavian vein and collateral circulation towards the right atrium was visualized (Figures 2C and 2D). Endoscopic examination revealed grade II esophageal varices. She received intravenous heparin, and subsequently coumarin derivatives. However, the ascites still remained intractable. Therefore, the patient was treated with percutaneous balloon dilatation therapy for the stenosis in the orifice of her right accessory hepatic vein (Figures 2E and 2F). The pressure in the right accessory hepatic vein was reduced after this treatment from 12 to 5 mmHg. After this treatment, the ascites was subsequently reduced and disappeared 2 wk later. Up until now (1 year later), no recurrent ascites have been recognized.
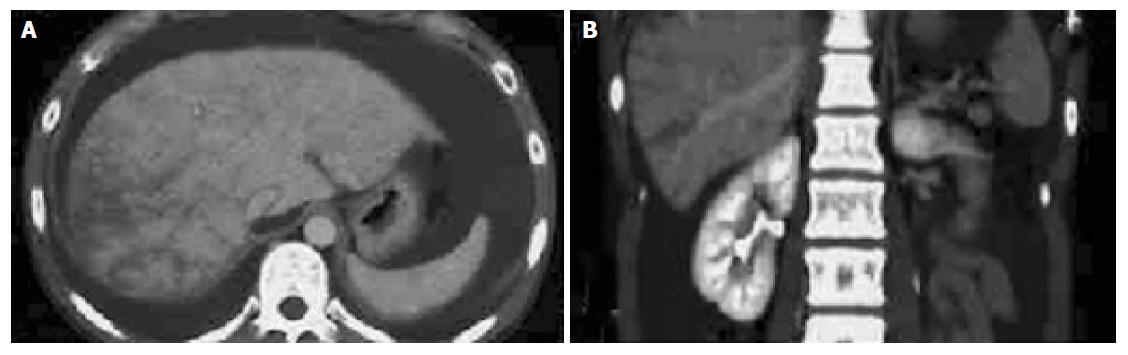
Figure 1 Computerized tomography scan of the abdomen.
(A) and (B) show the sagittal and coronal images of abdomen, respectively. Hepatomegaly with inhomogeneous distribution of contrast medium is visualized. Massive ascites is also recognized.
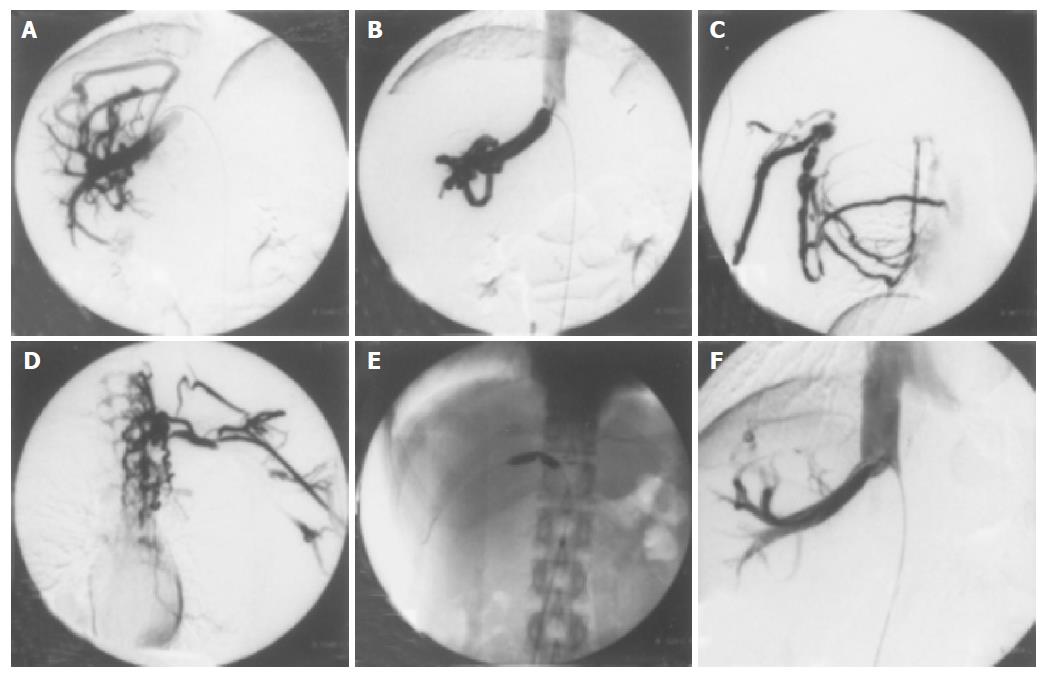
Figure 2 Cavography via the femoral vein and median basilic veins.
(A) and (B) show cavography via the femoral vein. Normal hepatic vein is not visualized and extensive collateral circulation (the right accessory hepatic vein) flows into the inferior vena cava. The orifice of this collateral vein is remarkably stenotic. (C) and (D) show cavography via right and left median basilic veins, respectively. Complete obstruction from the axillary to the subclavian vein and collateral circulation towards the right atrium are visualized. (E) shows a percutaneous balloon dilatation therapy for the stenosis in the orifice of her right accessory hepatic vein. (F) shows the dilated orifice of her right accessory hepatic vein after a balloon dilatation therapy.
DISCUSSION
BCS is a rare disease caused by hepatic vein obstruction. It leads to hepatic congestion, and consequently portal hypertension, ascites, a reduction in hepatic blood flow, and hepatocyte necrosis. Various etiologies for BCS such as idiopathic membranes[5], neoplasia[5], infection[6], trauma[7], and total parenteral nutrition[8] have been proposed. There is a well-defined association between BCS and the states of enhanced intravascular thrombosis such as paroxysmal nocturnal hemoglobinuria[3], the presence of the lupus anticoagulant[9] or a factor V Leiden mutation[10] and deficiencies of antithrombin III[11], protein C or protein S[12]. Similarly, myeloproliferative syndrome and, in particular, polycythemia vera are associated with an increased incidence of venous thrombosis in general, and with hepatic vein thrombosis in particular[2]. In 10-30% of patients with BCS, however, no clear cause can be identified[1]. In our case, all of the pathological entities mentioned above were ruled out. In particular, it has been reported that no Japanese person possesses the Leiden mutation[13], and in this case, no accelerated coagulation status was recognized. Therefore, we were unable to provide a definitive diagnosis in this case. Considering the occlusion of the superior vena cava in this case, an entirely different cause might be present in this case such as the congenital venous abnormalities. With respect to the treatment for BCS, conventional medical treatment with diuretics and anticoagulation has been useful, especially for patients at an early stage of acute thrombosis[14]. In this case, we chose diuretics and anticoagulation treatment. However, the ascites were intractable to these treatments. On the other hand, radiological interventions such as balloon angioplasty, metallic stent insertion and transjugular intrahepatic portosystemic shunts have been shown to be effective for selected patients with BCS. We chose balloon angioplasty, and obtained good clinical results. However, this treatment contains the risk of restenosis[15]. Therefore, we are carefully observing the clinical course of this patient. In particular, she is suffering from superior vena cava occlusion and may suffer the risk of some complications, such as edema in her arms or face, an elevation of intracranial pressure, and so on. Liver transplantation has been considered to be the best treatment for end stage or irreversible liver damage in BCS patients, especially in the Western world[16]. If deteriorating liver function or intractable ascites occurs in this case, a liver transplantation may be anticipated. In conclusion, we have described a young female patient with BCS, who did not have any specialized risk factors, and was suffering from a combination of hepatic vein and superior vena cava occlusion. To the best of our knowledge, this is the first reported case of BCS associated with hepatic vein and superior vena cava occlusion.
Science Editor Guo SY Language Editor Elsevier HK