Published online Jun 28, 2005. doi: 10.3748/wjg.v11.i24.3772
Revised: December 9, 2004
Accepted: January 5, 2005
Published online: June 28, 2005
AIM: Persistent hepatitis B virus (HBV) infection is characterized by a weak CD8+ T cell response to HBV. Immunotherapeutic strategies that overcome tolerance and boost these suboptimal responses may facilitate viral clearance in chronically infected individuals. Therefore, we examined whether CD25+CD4+ regulatory T (Treg) cells might be involved in a inhibition of CD8+ T cell priming or in the modulation of the magnitude of the ‘peak’ antiviral CD8+ T cell response primed by DNA immunization.
METHODS: B10.D2 mice were immunized once with plasmid pCMV-S. Mice received 500 μg of anti-CD25 mAb injected intraperitoneally 3 d before DNA immunization to deplete CD25+ cells. Induction of HBV-specific CD8+ T cells in peripheral blood mononuclear cells (PBMCs) was measured by S28-39 peptide loaded DimerX staining and their function was analyzed by intracellular IFN-γ staining.
RESULTS: DNA immunization induced HBV-specific CD8+ T cells. At the peak T cell response (d 10), 7.1±2.0% of CD8+ T cells were HBV-specific after DNA immunization, whereas 12.7±3.2% of CD8+ T cells were HBV-specific in Treg-depleted mice, suggesting that DNA immunization induced more antigen-specific CD8+ T cells in the absence of CD25+ Treg cells (n = 6, P<0.05). Similarly, fewer HBV-specific memory T cells were detected in the presence of these cells (1.3±0.4%) in comparison to Treg-depleted mice (2.6±0.9%) on d 30 after DNA immunization (n = 6, P<0.01). Both IFN-γ production and the avidity of the HBV-specific CD8+ T cell response to antigen were higher in HBV-specific CD8+ T cells induced in the absence of Treg cells.
CONCLUSION: CD25+ Treg cells suppress priming and/or expansion of antigen-specific CD8+ T cells during DNA immunization and the peak CD8+ T cell response is enhanced by depleting this cell population. Furthermore, Treg cells appear to be involved in the contraction phase of the CD8+ T cell response and may affect the quality of memory T cell pools. The elimination of Treg cells or their inhibition may be important in immunotherapeutic strategies to control HBV infection by inducing virus-specific cytotoxic T lymphocyte responses in chronically infected subjects.
- Citation: Furuichi Y, Tokuyama H, Ueha S, Kurachi M, Moriyasu F, Kakimi K. Depletion of CD25+CD4+T cells (Tregs) enhances the HBV-specific CD8+ T cell response primed by DNA immunization. World J Gastroenterol 2005; 11(24): 3772-3777
- URL: https://www.wjgnet.com/1007-9327/full/v11/i24/3772.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i24.3772
Cytotoxic T lymphocytes (CTLs) contribute to the control of hepatitis B virus (HBV) infection by killing infected cells and by inhibiting viral replication[1-3]. Acutely infected patients exhibit a vigorous, polyclonal and multispecific CTL response to the viral proteins sufficient to clear the infection, whereas chronically infected patients produce a weak or undetectable CTL response to HBV[4-6]. The mechanisms responsible for T cell hyporesponsiveness or tolerance to HBV proteins in patients with chronic HBV infections are not completely understood. The knowledge of the factors contributing to hyporesponsiveness may influence immunotherapeutic regimens (e.g., vaccines) designed to overcome immunological tolerance and thereby, terminate persistent viral infections.
Effective prophylactic and/or therapeutic vaccines directed against viral infections should establish a long-lasting antiviral effector/memory CD8+ T cell response. Genetic vaccination, i.e., DNA vaccines, is one of the most potent techniques for priming MHC class I-restricted CD8+ T cell responses[7,8]. Major advantages of DNA vaccines include their flexibility and their ability to induce protective immunity in multiple animal models and humans[7,8]. However, DNA vaccines alone do not induce responses as strong as conventional vaccines, i.e., live or attenuated. Consequently, efforts to enhance the immunogenicity of DNA vaccines are warranted. In DNA vaccination, plasmid DNA is injected and transiently expressed in cells of the skin or muscle. We previously reported the priming of class I-restricted CD8+ T cell responses to the hepatitis B surface antigen (HBsAg) by the intramuscular inoculation of expression plasmid DNA[9]. In the current study, we employed DNA immunization in H-2d mice which primes CD8+ T cell responses to the Ld-binding peptide IPQSLPSWWTSL (residue 28-39) as a representative antigen.
The primary response to antigen consists of a massive burst of naïve CD8+ T cells followed by a contraction of the antigen-specific T cell population. The majority of antigen-specific T cells undergo apoptosis, while the stable memory population survives. Efficient vaccines should induce as large an effector T cell population as possible since the burst size determines the number of memory T cells[10]. Although expansion can be initiated by brief contact with antigen, but other factors may control the magnitude of the CD8+ T cell response[11-13]. The extent to which other factors contribute to the optimization of the CD8+ T cell response is unresolved.
CD25+CD4+ regulatory T (Treg) cells have been shown to negatively regulate CD8+ T cell responses[14,15]. For example, both immunopathological and protective immune responses to intracellular parasites and viruses are affected by CD25+CD4+ Treg cells[16-19]. Therefore, we hypothesized that CD25+CD4+ Treg cells may contribute to the inhibition of CD8+ T cell priming and that the elimination of Treg cell suppression may modulate the magnitude of the ‘peak’ antiviral CD8+ T cell response primed by DNA immunization.
Specific pathogen free, male B10.D2 mice were purchased from SLC Japan (Shizuoka, Japan). All animal procedures described in this study were approved by the institutional animal care and use committee and performed according to the guidelines for animal experiments of Tokyo Medical University.
A peptide corresponding to a known H-2d restricted HBsAg-specific CTL epitope (S28-39; IPQSLDSWWTSL) was synthesized (Sigma Genosis, Ishikari, Japan). A plasmid (pCMV-S) that expresses HBsAg under the transcriptional control of the CMV immediate early promoter was kindly provided by Aldevron (Fargo, ND)[20].
Mice were immunized once with plasmid pCMV-S; 50 µg of plasmid DNA was injected into regenerating tibialis anterior muscles 5 d after the injection of cardiotoxin[20]. Ten days after the immunization, HBV-specific CD8+ T cells in the peripheral blood mononuclear cell population (PBMCs) were examined for the induction of HBV-specific CD8+ T cells.
Anti-CD25 IL-2Rα mAb from hybridoma PC61 (ATCC) was used to deplete CD25+ cells. In vivo depletion was performed using a modification of the procedure of Onizuka et al[21]. Mice received 500 μg of anti-CD25 mAb injected intraperitoneally (IP) 3 d prior to DNA immunization. Immediately before vaccination, blood was collected and the PBMC population was analyzed to confirm the elimination of CD25+CD4+ T cells.
PBMCs were washed twice in phosphate-buffered saline (PBS) containing 1% BSA and 0.02% sodium azide. Nonspecific antibody binding to the Fc receptor was blocked by preincubating the cells in culture media supernatant from the hybridoma cell line 2.4G2 (HB-197 [American Type Culture Collection]). Cells were incubated in the presence of the relevant mAb (0.5 μg/106 cells) for 30 min at 4 °C and washed twice. Multicolor flow cytometry analysis was performed using a BD LSR II and the data were analyzed using CELLQuest software (Becton Dickinson Immunocytometry Systems). The following reagents and mAbs were obtained from BD Biosciences Pharmingen (San Diego, CA): fluorescein-isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated anti-CD4 mAb GK1.5, FITC-, PE- and allophycocyanin (APC)-conjugated anti-CD8 mAb 53-6.7 and APC-conjugated anti-CD25 mAb 7D4. DimerX Recombinant Soluble Dimeric Mouse H-2Ld Ig (BD Pharmingen) was used to detect HBV-specific CD8+ T cells. S28-39 peptide was loaded on DimerX according to the manufacturer’s instruction. Cells were incubated with FITC-conjugated anti-CD8 mAb and S28-39 peptide loaded DimerX for 2 h at 4 °C and washed twice. Cells were subsequently incubated with PE-conjugated anti-mouse IgG1 mAb A85-1 mAb (BD Pharmingen) for 30 min at 4 °C, washed twice and analyzed by multicolor flow cytometry.
PBMCs and spleen cells (5×105) were cultured in 200 μL of RPMI medium in the presence or absence of S28-39 peptide (1 μg/mL) in 96-well round-bottom plates. Fifty units per milliliter of human recombinant interleukin-2 and brefeldin A (1 μg/mL) were added to the cultures. After 5 h, the cells were harvested, washed in PBS (containing 1% BSA and 0.02% sodium azide), and incubated for 20 min on ice with culture supernatant from the hybridoma cell line 2.4G2. The cells were surface stained with FITC-conjugated monoclonal anti-mouse CD8 mAb for 20 min on ice. After washing to remove unbound antibody, the cells were stained with APC-conjugated anti-mouse IFN-γ antibody (clone XMG1.2) and its isotype control antibody (rat IgG1) using a Cytofix/Cytoperm kit (BD Pharmingen) and analyzed by flow cytometry.
Student’s t-test was used to compare the frequency of HBV-specific CD8+ T cells between groups. Statistical analyses were performed with JMP Software, version 5.1.1J (SAS Institute Inc., Cary, NC). P values less than 0.05 were considered to be indicative of a statistically significant difference.
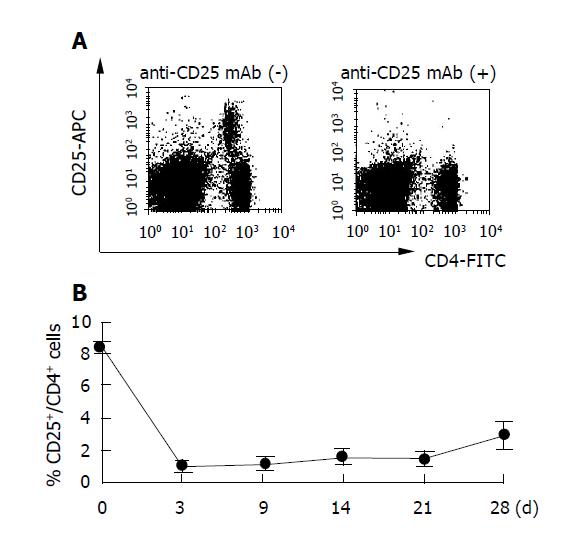
Naturally occurring Treg cells constitutively express IL-2Rα (CD25) and represent 5-10% of peripheral blood CD4+ cells in mice[22-24]. Since previous studies showed that anti-CD25 mAb depletes CD25+ cells in vivo, we injected 0.5 mg of anti-CD25 mAb IP into B10.D2 mice[21]. Three days later flow cytometry confirmed the extent of CD25+ cell depletion, i.e., 8.5±0.3% to 1.0±0.1% (Figure 1). The percentage of peripheral blood CD25+CD4+ T cells remained below 2% for more than 4 wk after anti-CD25mAb treatment (P<0.01 vs anti-CD25 mAb(-) group).
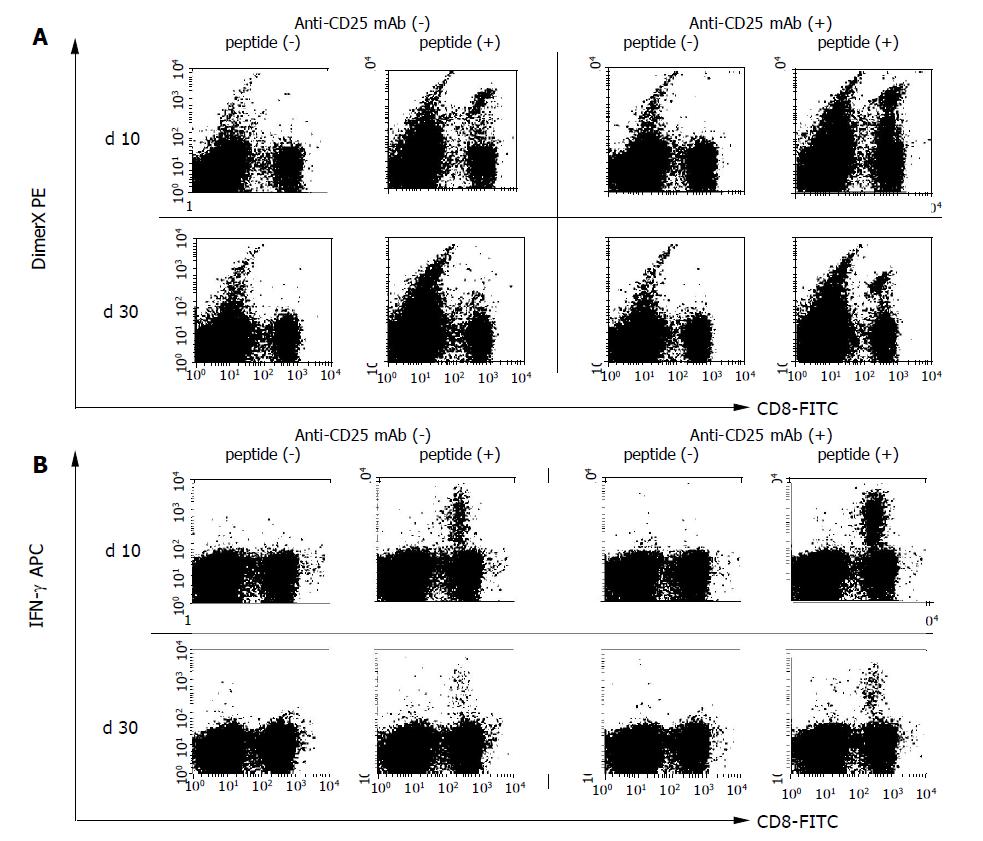
We immunized B10.D2 mice (six mice per group) by intramuscular injection of 100 μg of pCMV-S. Ten days after DNA immunization at the peak of the primary T cell response, PBMCs were examined for the induction of HBV-specific CD8+ T cells by S28-39 peptide loaded DimerX staining (Figure 2A). As shown in Figure 3A, 7.1±2.0% of the CD8+ T cell population was positive suggesting that DNA immunization induced HBV S28-39 specific CD8+ T cells in these animals.
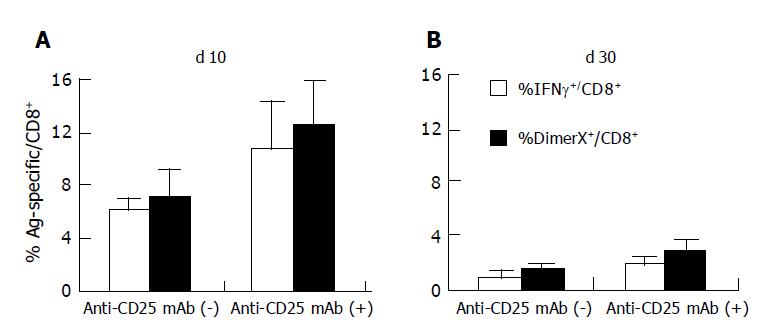
In order to test whether or not Treg cells control the induction and/or expansion of the antigen-specific T cell population following DNA immunization in vivo, Treg cells were depleted by IP injection of anti-CD25 mAb (500 μg). Three days later the PBMC population was analyzed to confirm the elimination of CD25+CD4+ T cells (Figure 1). Mice exhibiting CD25+ cell depletion were further immunized with 100 μg of pCMV-S DNA. Ten days after DNA immunization, the induction of S28-39 specific CD8+ T cells was measured in the PBMC population using S28-39 peptide loaded DimerX. As shown in Figures 2 and 3A, 12.7±3.2% of the CD8+ T cells in anti-CD25 mAb-treated mice were specific for HBV S28-39 peptide. These data suggest that DNA immunization induced more antigen-specific CD8+ T cells in the absence of CD25+ T cells and/or that the expansion of the HBV S28-39 specific T cell population was greater in the CD25+ depleted mice than in the non-depleted animals, i.e., 7.1±2.0%, n = 6, P<0.05. These results suggest that CD25+ Treg cells suppress priming and/or expansion of antigen-specific CD8+ T cells in DNA immunization and that the peak CD8+ T cell response can be enhanced by the depletion of this cell population.
We observed a marked expansion of the HBV-specific CD8+ T cell population 10 d after DNA immunization. This should be followed by a subsequent contraction of antigen-specific T cell population. This process is critical for the generation of stable memory cells. In order to assess the efficacy of this process, we compared the HBV-specific CD8+ T cell response in CD25+ depleted and non-depleted mice 30 d after DNA immunization (Figures 2 and 3B). As expected, twice as many HBV-specific CD8+ T cells were observed in CD25+ depleted animals compared to non-depleted animals (n = 6, P<0.01). The frequency of HBV S28-39 specific CD8+ T cells decreased from 7.1±2.0% to 1.3±0.4% in the control animals by d 30, suggesting that 17.7% of the HBV S28-39 specific CD8+ T cells survived as memory T cells. In contrast, the CD25+ depleted mice exhibited a five-fold decrease between d 10 and 30, i.e., 12.7±0.3% vs 2.6±0.9% in the CD8+ T cell population. Approximately 20.5% of the HBV S28-39 specific CD8+ T cell population remained in the CD25+ depleted mice, suggesting that survival ratio of antigen-specific T cells was comparable in the presence and absence of these regulatory cells during the contraction phase.
PBMCs were harvested and stimulated with S28-39 peptide in order to examine cytokine production by S28-39 specific CD8+ T cells following DNA immunization (Figures 2B and 3). In the control mice, 6.1±0.6% of the CD8+ T cells produced IFN-γ, suggesting that 90.6% of the HBV S28-39 specific CD8+ T cells were functional. In the CD25+ depleted mice, 10.7±3.6% of the CD8+ T cells produced IFN-γ on d 10 following DNA immunization suggesting that almost all of the HBV S28-39 specific CD8+ T cells (88.3% of DimerX positive cells) produced IFN-γ (Figure 3A). Therefore, HBV-specific CD8+ T cells induced by DNA immunization were equivalent with respect to cytokine production at the peak of the T cell response regardless of the presence or absence of CD25+CD4+ Treg cells.
Some of the antigen-specific T cells lost their effector functions during the contraction phase. In the control mice, 0.8% of CD8+ T cells produced IFN-γ in response to S28-39 peptide and 1.3±0.4% of these cells were DimerX positive, suggesting that -66% of the HBV S28-39 specific T cells were functional (Figure 3B). In contrast, -70% of the HBV S28-39 specific T cells were functional by d 30 in the CD25+ depleted mice, i.e.,1.7% IFN-γ positive and 2.6% DimerX positive (Figure 3B). These results suggest that antigen-specific T cells maintain their cytokine producing capacity during the contraction phase regardless of the presence or absence of CD25+CD4+ Treg cells.
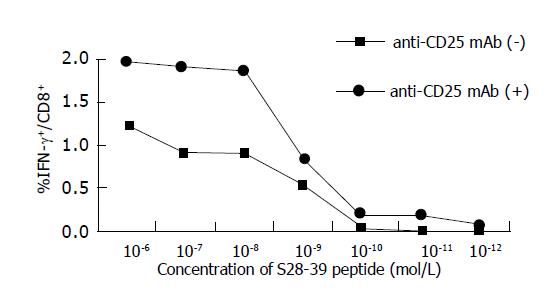
The avidities of HBV S28-39 specific CD8+ T cells were compared in a peptide dose titration experiment monitoring IFN-γ secretion after stimulation with epitope peptides (Figure 4). PBMCs were harvested 40 d after DNA immunization and incubated in the presence of S28-39 epitope (10-12 to 10-6 mol/L) for 6 h. HBV-specific CD8+ T cells induced in both control and CD25+ depleted mice displayed 50% of maximal IFN-γ production at a peptide concentration of 3×10-9 mol/L. These data suggest that the avidities of HBV-specific CD8+ T cells induced by DNA immunization were comparable in control and CD25+ depleted animals. Interestingly, CD8+ T cells only in the CD25+ Treg cell depleted mice were able to respond to S28-39 peptide at as low as 10-11 mol/L, suggesting that CD8+ T cells with higher avidity can be induced by DNA immunization only in the absence of CD25+CD4+ Treg cells.
The intensity of many immune inflammatory reactions is subject to control by Treg cells[25]. Although initial studies focused on organ-specific autoimmunity, immunopathological reactions to foreign antigens are also influenced by Treg cells[16,26,27]. This report assessed the role of CD25+CD4+ Treg cells in regulating the T cell response to DNA immunization and their effect on the development of antigen-specific memory T cells.
We demonstrated that the magnitude of a T cell response to DNA immunization is subject to control by CD25+CD4+ Treg cells. Depleting Treg cells with anti-CD25 mAb prior to DNA immunization significantly enhanced the CD8+ T cell response to the S28-39 peptide. Furthermore, CD25+ Treg-depleted mice exhibited a 50% increase in the peak CD8+ T cell response following DNA immunization (Figure 2). Consequently, more HBV-specific memory CD8+ T cells were produced in CD25+ Treg-depleted mice. The ability of T cells to secrete IFN-γ following antigen stimulation was equivalent between CD25+ Treg depleted and control mice. More importantly, HBV-specific CD8+ T cells exhibiting high functional avidity were induced in the absence of CD25+ Treg cells by DNA immunization to a greater extent than were control cells.
A brief exposure to antigen is sufficient for naïve CD8+ cytotoxic T cell precursors to initiate the process that culminates in multiple rounds of cell division and leads to the acquisition of effector function and the creation of a memory T cell pool[11-13]. However, this does not mean that the program is entirely independent of extrinsic regulatory factors, but only that the TCR on CD8+ T cells does not require further association with foreign antigen to initiate this cascade of events. Other factors may contribute to the optimization of the CD8 response, but they remain unidentified. These observations provide a potential opportunity to intervene in this sequence of events in order to enhance vaccine efficacy. In this study, we demonstrated that Treg cells contribute to the regulation of the magnitude of the CD8+ T cell response and, therefore, the depletion of this cell population may permit the modulation of this process.
Our data may have an impact on vaccine development since effective immunotherapies capable of terminating chronic viral infections are still unavailable. Viral antigens expressed during the chronic carrier stage provide ideal targets for antigen-specific immunotherapy. However, immunotherapy for established chronic infections does not yet have widespread clinical applicability. The marked differences in the success of prophylaxic vs therapeutic vaccines reflect the fact that pathogens have developed mechanisms to avoid recognition and elimination by the immune system. The induction of antigen-specific tolerance via normal tolerogenic pathways is the mechanism utilized by viruses to avoid recognition. It is reported that the expression of viral antigen itself plays an active role in the induction of antigen-specific tolerance[9]. Reactivation and expansion of residual antigen-specific T cells that are either of low affinity or specific for epitopes presented at low density are of particular importance in the design of therapeutic vaccines targeted at patients with existing infections[9]. Conventional strategies that deliver antigens by vectors are not effective, since high levels of pre-existing antigen impair T cell responses. Efficient antiviral requires that the patient’s antigen levels are sufficiently diminished long enough to permit (a) thymic production of high affinity antigen-specific T cells and (b) peripheral T cells to survive and display full antiviral effector function. We have demonstrated that DNA immunization in conjunction with anti-CD25 mAb depletion of Treg cells induced HBV-specific CD8+ T cells with high avidity TCR that recognize diminished expression of viral antigen. Furthermore, our study clearly demonstrates that DNA immunization with anti-CD25 mAb treatment increases the magnitude of the HBV-specific CD8+ T cell response. More HBV-specific CD8+ T cells were induced and they exhibited a higher avidity to antigens. Expansion of the HBV-specific CD8+ T cell population resulted in more memory T cells in Treg cell-depleted animals. Although it remains to be elucidated whether this vaccine protocol can overcome T cell tolerance in chronically HBV infected individuals, our data encourage the development of immunotherapeutic regimens for chronic HBV infection. Novel vaccine strategies that may help T cells regain their responsiveness and full effector function may be achieved by depleting the Treg cell population and/or suppressing their function.
We thank Yasuhiko Koezuka for helpful discussion and Yasuko Uehara for excellent technical assistance.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1201] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 2. | Guidotti LG, Chisari FV. To kill or to cure: options in host defense against viral infection. Curr Opin Immunol. 1996;8:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 182] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Chisari FV. Rous-Whipple Award Lecture. Viruses, immunity, and cancer: lessons from hepatitis B. Am J Pathol. 2000;156:1117-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 229] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Rehermann B, Fowler P, Sidney J, Person J, Redeker A, Brown M, Moss B, Sette A, Chisari FV. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med. 1995;181:1047-1058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 368] [Cited by in RCA: 380] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 5. | Bertoletti A, Chisari FV, Penna A, Guilhot S, Galati L, Missale G, Fowler P, Schlicht HJ, Vitiello A, Chesnut RC. Definition of a minimal optimal cytotoxic T-cell epitope within the hepatitis B virus nucleocapsid protein. J Virol. 1993;67:2376-2380. [PubMed] |
| 6. | Nayersina R, Fowler P, Guilhot S, Missale G, Cerny A, Schlicht HJ, Vitiello A, Chesnut R, Person JL, Redeker AG. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J Immunol. 1993;150:4659-4671. [PubMed] |
| 7. | Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 868] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 8. | Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization*. Annu Rev Immunol. 2000;18:927-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 867] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 9. | Kakimi K, Isogawa M, Chung J, Sette A, Chisari FV. Immunogenicity and tolerogenicity of hepatitis B virus structural and nonstructural proteins: implications for immunotherapy of persistent viral infections. J Virol. 2002;76:8609-8620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1245] [Cited by in RCA: 1373] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 11. | van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naïve CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423-429. [PubMed] |
| 12. | Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol. 2001;2:415-422. [PubMed] |
| 13. | Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165:6833-6839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 380] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 14. | Kursar M, Bonhagen K, Fensterle J, Köhler A, Hurwitz R, Kamradt T, Kaufmann SH, Mittrücker HW. Regulatory CD4+CD25+ T cells restrict memory CD8+ T cell responses. J Exp Med. 2002;196:1585-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Murakami M, Sakamoto A, Bender J, Kappler J, Marrack P. CD25+CD4+ T cells contribute to the control of memory CD8+ T cells. Proc Natl Acad Sci USA. 2002;99:8832-8837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 177] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889-901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 415] [Cited by in RCA: 430] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 17. | Hori S, Carvalho TL, Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol. 2002;32:1282-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1308] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 19. | Xu D, Liu H, Komai-Koma M, Campbell C, McSharry C, Alexander J, Liew FY. CD4+CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J Immunol. 2003;170:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 184] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Davis HL, Schirmbeck R, Reimann J, Whalen RG. DNA-mediated immunization in mice induces a potent MHC class I-restricted cytotoxic T lymphocyte response to the hepatitis B envelope protein. Hum Gene Ther. 1995;6:1447-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128-3133. [PubMed] |
| 22. | Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151-1164. [PubMed] |
| 23. | Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212-1218. [PubMed] |
| 24. | Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1240] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 25. | Fehérvari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest. 2004;114:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 281] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 26. | Rouse BT, Suvas S. Regulatory cells and infectious agents: detentes cordiale and contraire. J Immunol. 2004;173:2211-2215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123-4132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 274] [Article Influence: 13.0] [Reference Citation Analysis (0)] |