Published online Jun 28, 2005. doi: 10.3748/wjg.v11.i24.3762
Revised: November 22, 2004
Accepted: December 20, 2004
Published online: June 28, 2005
AIM: Celiac disease (CD) is an enteropathic disorder very prevalent in Saharawi people. Our aim was to investigate the diagnostic accuracy of six human tissue transglutaminase (tTG) based ELISA tests in Saharawi CD patients.
METHODS: Fifty-two CD patients and 23 controls were selected from the Saharawi refugee camps in Tinduf. CD patients were divided into two groups according to their anti-endomysium (EmA) status: 41 EmA positive and 11 EmA negative. Sera from patients and controls were tested for human tTG using six commercial ELISA kits. We used receiver operating characteristics (ROC) curves and areas under the curve to compare the diagnostic accuracies of the six assays.
RESULTS: In general, there are differences in the sensitivity and specificity of the human tTG ELISA assays used. Diagnostic accuracy of tests was significantly improved by adjusting the cut-off thresholds according to ROC plot analysis; the correction of the cut-off with the employment of the ROC curve analysis modifies the decision limit in more than 50% in five of the six kits evaluated.
CONCLUSION: Some of the human tTG ELISAs used in this study have a diagnostic accuracy similar to EmA determination for diagnosis of CD in Saharawi people. However, it is necessary to select the assay with a higher sensitivity and specificity, and recalculate the cut-off threshold using samples from the referral population.
- Citation: Fernández E, Riestra S, Rodrigo L, Blanco C, López-Vázquez A, Fuentes D, Moreno M, López-Larrea C. Comparison of six human anti-transglutaminase ELISA-tests in the diagnosis of celiac disease in the Saharawi population. World J Gastroenterol 2005; 11(24): 3762-3766
- URL: https://www.wjgnet.com/1007-9327/full/v11/i24/3762.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i24.3762
Celiac disease (CD) is a gluten-sensitive enteropathy occurring in genetically susceptible individuals. The disease is associated with HLA alleles DQα1*0501/DQβ1*0201[1]. CD is a worldwide disorder, and gluten intake and genetic predisposition are the two main factors involved in its frequency in a population[2]. In Europe or America, the prevalence of CD is 0.3-1% of the general population[3-7]. The highest frequency of CD in the world has been reported in Saharawi children (5.6%)[8]. Genetic and environmental factors could explain this remarkable finding; thus, the DR3/ DQα1*0501/DQβ1*0201 haplotype is present in 40% of the Saharawi general population[9,10] and there is an early introduction of gluten in the diet of Saharawi children and a high consumption of cereals.
In the general population, most of the patients affected by CD have a silent or atypical clinical presentation, and a non-invasive test is therefore necessary to select subjects for a biopsy of the small intestine. Serological screening with IgA anti-endomysium antibodies (EmA) has been considered the gold standard of laboratory diagnostic procedures. However, EmA are tested using an indirect immuno-fluorescence method, which is a time-consuming and subjective technique[11]. Tissue transglutaminase (tTG) has been identified as the endomysial autoantigen of CD[12], and an enzyme linked immuno-absorbent assay (ELISA) for the detection of serum antibodies against tTG has been developed[13]. At present, the tTG-based ELISA is the method of choice for serological screening of CD[14,15], because ELISA techniques are less expensive and less subjective than immuno-fluorescence methods.
Human tTG ELISAs have replaced guinea pig tTG based assays due to a better correlation with EmA as an indicator of gluten-sensitive enteropathy[16]. However, discrepancies in the results have been found when several human tTG ELISA tests were compared[17-19]; thus, it is necessary to verify the applicability of human tTG based assays, in routine clinical practice. On the other hand, false positive results have been reported in patients with chronic liver disease[20] and with end-stage heart failure[21]. It is not known whether there are other processes that could influence the diagnostic accuracy of human tTG ELISAs.
Saharawi people live in an undeveloped region, where the accessibility to different medical technologies (for example, digestive endoscopy) is very difficult. Thus, to possess an easy and cheap method in order to diagnose a highly prevalent disease such as CD could have an important impact on the health status of this population. The aim of this study was to verify the diagnostic accuracy of six commercially human tTG ELISAs in both Saharawi controls and EmA-positive CD patients.
Fifty-two Saharawi patients randomly selected from 125 celiac previously reported patients[10] were included in the study. All patients were recruited by two members of our group in the Tinduf refugee camps, during a medical visit, made in May 2002. Twenty-three untreated, biopsy confirmed CD patients diagnosed previously, and 18 new patients classified as CD patients on being positive for EmA, prior to starting on a gluten-free diet (GFD), were used as the disease group. Serum samples from 11 CD patients on a GFD were also analyzed for EmA and tTG antibodies. All CD patients were classified into typical and atypical forms according to their clinical manifestations[2]. From the 52 CD patients, 29 had a classical digestive symptomatology, consisting of diarrhea, flatulence, weight loss and fatigue; these patients were classified as typical forms of the CD. Twenty-three had extra-intestinal, single-predominant symptoms, or were even symptomless, and were thus classified as atypical forms; most of them had ferropenic anemia as the predominant manifestation and were diagnosed only by the presence of EmA antibodies.
Twenty-three healthy controls from the Saharawi population, all of whom were without direct familiar relationship with CD patients, without a history of intolerance to gluten and derivatives, without iron deficiency, ferropenic anemia or any abnormal finding in the biochemical studies and with negativity for EmA, were included in the study.
The subjects of the study were grouped according to the EmA status: healthy controls (group I); EmA positive CD patients (includes new and known untreated CD patients) (group II); and EmA negative CD patients on GFD (group III).
The Tindouf Health Authority of Saharawi granted permission for the study, and all patients and controls gave informed consent prior to enrollment.
Serum IgA EmA assay In all patients and controls IgA class EmA values were determined with a commercially available indirect immuno-fluorescence method using slides of monkey esophagus (BioSystems, supplied by Atom, Barcelona, Spain) at a screening dilution of 1/5 as recommended by the manufacturer. EmA negative sera from known CD patients were further tested at a 1/2 dilution to exclude false negative results. Based on the EmA results, the subjects of the study were classified in three groups, as described above.
Five milliliters of blood serum were obtained from each individual and tTG antibodies were determined by six commercially available sandwich ELISAs that use human antigen obtained from different sources: Orgentec, Diagnostika GmbH, (Mainz, Germany), human recombinant tTG from human embryonic kidney cell line 293-EBNA; Celikey, Pharmacia Diagnostics GmbH (Freiburg, Germany), human recombinant tTG from eukaryotic cells of Lepidoptera (Baculovirus/Sf9 system); Quanta Lite, Inova Diagnostics (San Diego, CA, USA), purified native red blood cell tTG; AESKULISA, Aesku.lab Diagnostka (Wendelsheim, Germany), human recombinant tTG from human embryonic kidney cell line 293-EBNA; Eurospital SpA (Trieste, Italy), human recombinant tTG from E.coli; and BioSystems (Barcelona, Spain), human recombinant tTG from E.coli. All measurements were made in a unique batch on a Triturus ELISA autoanalyser (Grifols) by a single operator following the manufacturer’s instructions. Intra and interassay CVs for the six human tTG ELISAs were below 10%.
To exclude IgA deficiency, total serum IgA concentrations were also measured in all CD patients and healthy controls by nephelometry (BN II, Dade-Behring, Frankfurt, Germany). No cases yielded IgA levels below 0.05 g/L, indicative of selective IgA deficiency, and the values obtained were within the reference range (>1.24 g/L).
Two milliliters of peripheral blood was obtained from each patient and control, and was mixed with 2 mL of ethanol and stored until the specimens were processed. Ethanol was removed by washing twice with PBS (Phosphate buffered saline). Finally genomic DNA was obtained with NUCLEOSPIN Blood LTM kits from MACHEREY-NAGEL, Düren, Germany. All patients and healthy controls were typed by PCR-SSP for HLA-DQA1 and for HLA-DQB1*0201/02 ambiguity according to a previously described method[22].
Descriptive analyses were used to characterize the study population. The Fisher exact test was used to compare dichotomous variables, and the unpaired Wilcoxon test was used to compare differences in the medians of the continuous nonparametric variables.
The diagnostic performance of each human tTG ELISA test or the ability to discriminate diseased from normal cases was evaluated using Receiver Operating Characteristic (ROC) curve analysis, thus selecting the cut-offs that provide the best combination of sensitivity and specificity. The areas under the ROC curves and their 95% confidence intervals (CI) were also calculated using the nonparametric method described by Hanley and McNeil[23], developed as a statistical software package (MedCal v. 7.2.1.0). For all statistical analyses, a two-tailed P<0.05 was considered as significant.
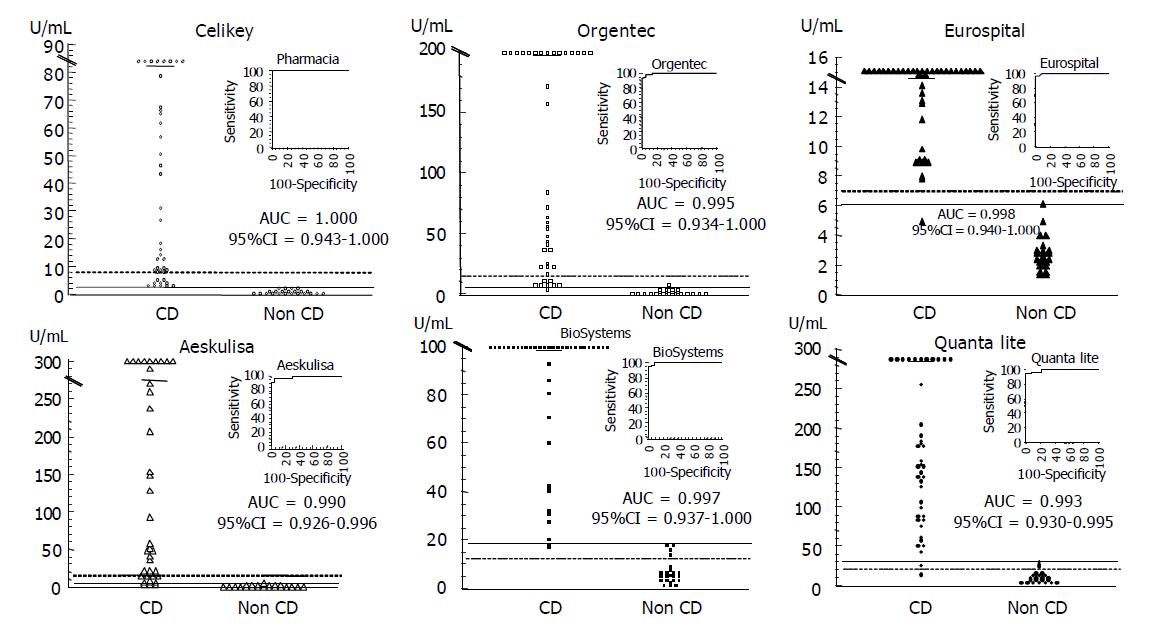
Demographic, clinical, serological and HLA characteristics of the 75 patients studied are summarized in Table 1. The IgA tTG values of the EmA-positive CD patients and the healthy controls measured with the six human tTG ELISA kits are shown in Figure 1. It is noteworthy that the correction of the cut-off with the employment of the ROC curve analysis modifies the decision limit in more than 50% in five of the six kits evaluated, with the exception of Eurospital. On the basis of the cut-offs by the manufacturers the human tTG ELISA kits assessed showed three patterns: low sensitivity (75.6-80.5%) and high specificity (100%) (Orgentec, AESKULISA, Celikey); high sensitivity (97.6-100%) and low specificity (82.6-87%) (BioSystems, Quanta Lite); and high sensitivity (97.6%) and specificity (100%) (Eurospital). Even when using the ROC curves cut-offs, five kits (Orgentec, AESKULISA, BioSystems, Quanta Lite, Eurospital) yielded false negatives, and two kits (Orgentec, AESKULISA) also showed false positives. Only the Celikey kit achieved 100% sensitivity and 100% specificity, in concordance with EmA results.
| Group I (n = 23) | Group II (n = 41) | Group III (n = 11) | |
| Female sex | 15 (65) | 29 (71) | 9 (82) |
| Age (yr) | |||
| Mean±SD | 20±15 | 17±12 | 16±11 |
| Range | 4-54 | 2-52 | 4-40 |
| Typical CD | 0 | 22 (46) | 7 (64) |
| DQA1*0501/DQB1*0201 | 8(35) | 39 (92) | 10 (91) |
| EmA positive | 0 | 41 (100) | 0 |
In the 11 EmA negative CD patients on a GFD (Group III) the levels of tTG antibodies (median, range, U/mL) were higher than those of healthy controls (Orgentec: 4.89, 2.5-7.9 vs 2.43, 0.52-7.45 P<0.01; AESKULISA: 3.36, 1.88-6.50 vs 1.64, 0.22-4.73 P<0.01; BioSystems: 16.2, 8.96-19.2 vs 5.12, 1.31-18.7 P<0.001; Quanta-Lite: 32.3, 11.8-43.7 vs 10, 4.1-30 P<0.001; Celikey, 1.84, 1.29-2.42 vs 0.79, 0.18-2.44 P<0.01; and Eurospital: 5.6, 4.4-6.8 vs 2.6, 1.3-6.1 P<0.001, respectively). The rate of positive human tTG cases in group III varied with both the human tTG ELISA and the criteria used to establish the cut-off (manufacturer or ROC curve). With the usual cut-offs the six human tTG ELISAs yielded the following tTG-positive rates: Orgentec, 0%; AESKULISA, 0%; BioSystems, 81.8%; Quanta-Lite, 72.7%; Celikey, 0%; and Eurospital, 0%. These figures were modified with the threshold on the basis of ROC curves analysis: Orgentec, 36.4%; AESKULISA, 27.3%; BioSystems, 18.2%; Quanta-Lite, 63.6%; Celikey, 0%; and Eurospital, 18.2%. Therefore, in the three groups studied, the Celikey kit was the only human tTG ELISA test that kept total concordance with EmA results when the decision threshold was established by ROC curves.
CD is a common disorder among the Saharawi population, with an important clinical and nutritional impact on their health status. Thus, a specific program for diagnosing and treating all affected individuals has been recommended[24]. EmA is a good marker of gluten-sensitive enteropathy in Saharawi children[8], but it is an expensive and subjective assay. Human tTG ELISA test, is similar to EmA assay, with regard to sensitivity and specificity[14,15], but most of the studies have been performed in the Caucasians. To our knowledge, this is the first study undertaken to compare several human tTG ELISA kits in Saharawi people, that is in both healthy controls and celiac patients.
A possible limitation of our study could be the lack of intestinal biopsies in the newly diagnosed patients. However, we believe that these subjects really have an enteropathy due to sensitivity to gluten, since all were EmA positive and 90% presented the HLA DQ2. Thus, it is known that the presence of EmA in the Saharawi population is associated with the existence of an enteropathy due to sensitivity to gluten[8] and that a frequency of HLA DQ2 between 88% and 91.3% has been reported in Saharawi celiac patients[9,10]. On the other hand, the predominant form of clinical presentation among the Saharawi celiac population is the classic or typical type diarrhea, abdominal distension[10, 24], similar to that which occurs in our work.
As previously described in Caucasians[17,19], differences were found in the sensitivity and specificity of the human tTG ELISA assays used. Diagnostic accuracy of tests was significantly improved by adjusting the cut-off thresholds according to ROC plot analysis; the importance of cut-off revalidation using appropriate samples from the referral population has been previously reported[19]. In our study, using the manufacturers’ cut-off thresholds in the Celikey assay (8 AU/mL], the sensitivity was low (75.6 %), while using the new cut-off value derived from ROC curves (2.45 AU/mL), the diagnostic accuracy of the test will be significantly improved (sensitivity and specificity of 100%). Similar to our results in Saharawi people, it has been reported that the best cut-off threshold for Celikey were 2.3 and 3 among the Italian and Spanish population, respectively[17,25]. We believe that the improvement in the sensitivity of human tTG ELISA tests when using the ROC-based cut-offs is due to adaptation of the test to our referral population.
In our study, we believe that the majority of the tTG positive/EmA negative results obtained with some kits in CD patients on GFD, are not really false positives since it has been seen that the negativization of the EmA after starting on a GFD does not correlate exactly with the disappearance of the intestinal lesion[26]. On the other hand, it has been observed that a discrepancy exists in the results of EmA and tTG antibodies when they are determined simultaneously during follow-up of the celiac patients treated. Thus, even when a severe enteropathy persists (Marsh 3 villous atrophy), the EmA tend to negativize before the tTG antibodies[27]. The presence of positive tTG cases in the healthy controls has been very scarce (Figure 1); although we do not possess an intestinal biopsy in order to rule out CD in these subjects, we believe that the concordance of the rest of the serological tests used (human tTG and EmA) and the absence of symptomatology, makes it improbable that we are dealing with unknown celiac patients. On the other hand, even when using human tGT assays, several positive cases have been described in the absence of CD[20,21].
In our study we have demonstrated that the determination of human tTG by means of ELISA is a method as precise as the detection of EmA by immunofluorescence, for the diagnosis of CD in the Saharawi population. In Saharawi children the presence of diarrhea, intestinal distension and retardation in growth is highly frequent[24], even though only 40% of these are going to suffer from CD as a cause of this symptomatology[28]; the rest of the cases will be caused by intestinal infections, or by chronic malnutrition. Thus, to possess a highly precise diagnostic test, which is easy to apply and relatively cheap, would provide an important tool for the early diagnosis and treatment of CD in this population, in which considerable difficulty exists in performing an intestinal biopsy. Some authors have demonstrated that the human tTG-based ELISA represents a cost-effective strategy for identifying both symptomatic and atypical forms of CD and this could mean that an intestinal biopsy need no longer be the gold standard for diagnosing this disorder[29].
Even though there were differences in sensitivity and specificity among the human tTG ELISAs used in this study, we can conclude that some of them have a diagnostic accuracy similar to EmA determination for diagnosis of CD in Saharawi people. However, it is necessary to select the ELISA kit with a higher sensitivity and specificity, and recalculate the cut-off threshold using samples from the referral population.
We wish to thank to Palex, Pharmacia, Menarini, Grifols, Innogenetics and Biosystems Laboratories, for providing to us the commercial kits of h-tTG used in this study.
We also thank David H Wallace (Member of the Council of Science Editors) for his critical revision of the English version of the manuscript.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Sollid LM, Thorsby E. HLA susceptibility genes in celiac disease: genetic mapping and role in pathogenesis. Gastroenterology. 1993;105:910-922. [PubMed] |
| 2. | Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001;120:636-651. [PubMed] [DOI] [Full Text] |
| 3. | Riestra S, Fernández E, Rodrigo L, Garcia S, Ocio G. Prevalence of Coeliac disease in the general population of northern Spain. Strategies of serologic screening. Scand J Gastroenterol. 2000;35:398-402. [PubMed] [DOI] [Full Text] |
| 4. | Volta U, Bellentani S, Bianchi FB, Brandi G, De Franceschi L, Miglioli L, Granito A, Balli F, Tiribelli C. High prevalence of celiac disease in Italian general population. Dig Dis Sci. 2001;46:1500-1505. [PubMed] [DOI] [Full Text] |
| 5. | Gomez JC, Selvaggio G, Pizarro B, Viola MJ, La Motta G, Smecuol E, Castelletto R, Echeverria R, Vazquez H, Mazure R. Value of a screening algorithm for celiac disease using tissue transglutaminase antibodies as first level in a population-based study. Am J Gastroenterol. 2002;97:2785-2790. [PubMed] [DOI] [Full Text] |
| 6. | Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, Elitsur Y, Green PH, Guandalini S, Hill ID. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286-292. [PubMed] [DOI] [Full Text] |
| 7. | Mäki M, Mustalahti K, Kokkonen J, Kulmala P, Haapalahti M, Karttunen T, Ilonen J, Laurila K, Dahlbom I, Hansson T. Prevalence of Celiac disease among children in Finland. N Engl J Med. 2003;348:2517-2524. [PubMed] [DOI] [Full Text] |
| 8. | Catassi C, Rätsch IM, Gandolfi L, Pratesi R, Fabiani E, El Asmar R, Frijia M, Bearzi I, Vizzoni L. Why is coeliac disease endemic in the people of the Sahara? Lancet. 1999;354:647-648. [PubMed] [DOI] [Full Text] |
| 9. | Catassi C, Doloretta Macis M, Rätsch IM, De Virgiliis S, Cucca F. The distribution of DQ genes in the Saharawi population provides only a partial explanation for the high celiac disease prevalence. Tissue Antigens. 2001;58:402-406. [PubMed] [DOI] [Full Text] |
| 10. | López-Vázquez A, Fuentes D, Rodrigo L, González S, Moreno M, Fernández E, Martínez-Borra J, López-Larrea C. MHC class I region plays a role in the development of diverse clinical forms of celiac disease in a Saharawi population. Am J Gastroenterol. 2004;99:662-667. [PubMed] [DOI] [Full Text] |
| 12. | Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797-801. [PubMed] [DOI] [Full Text] |
| 13. | Dieterich W, Laag E, Schöpper H, Volta U, Ferguson A, Gillett H, Riecken EO, Schuppan D. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology. 1998;115:1317-1321. [PubMed] [DOI] [Full Text] |
| 14. | Bürgin-Wolff A, Dahlbom I, Hadziselimovic F, Petersson CJ. Antibodies against human tissue transglutaminase and endomysium in diagnosing and monitoring coeliac disease. Scand J Gastroenterol. 2002;37:685-691. [PubMed] [DOI] [Full Text] |
| 15. | Tesei N, Sugai E, Vázquez H, Smecuol E, Niveloni S, Mazure R, Moreno ML, Gomez JC, Mauriño E, Bai JC. Antibodies to human recombinant tissue transglutaminase may detect coeliac disease patients undiagnosed by endomysial antibodies. Aliment Pharmacol Ther. 2003;17:1415-1423. [PubMed] [DOI] [Full Text] |
| 16. | Carroccio A, Vitale G, Di Prima L, Chifari N, Napoli S, La Russa C, Gulotta G, Averna MR, Montalto G, Mansueto S. Comparison of anti-transglutaminase ELISAs and an anti-endomysial antibody assay in the diagnosis of celiac disease: a prospective study. Clin Chem. 2002;48:1546-1550. [PubMed] |
| 17. | Martini S, Mengozzi G, Aimo G, Pagni R, Sategna-Guidetti C. Diagnostic accuracies for celiac disease of four tissue transglutaminase autoantibody tests using human antigen. Clin Chem. 2001;47:1722-1725. [PubMed] |
| 18. | Blackwell PJ, Hill PG, Holmes GK. Autoantibodies to human tissue transglutaminase: superior predictors of coeliac disease. Scand J Gastroenterol. 2002;37:1282-1285. [PubMed] [DOI] [Full Text] |
| 19. | Wong RC, Wilson RJ, Steele RH, Radford-Smith G, Adelstein S. A comparison of 13 guinea pig and human anti-tissue transglutaminase antibody ELISA kits. J Clin Pathol. 2002;55:488-494. [PubMed] [DOI] [Full Text] |
| 20. | Vecchi M, Folli C, Donato MF, Formenti S, Arosio E, de Franchis R. High rate of positive anti-tissue transglutaminase antibodies in chronic liver disease. Role of liver decompensation and of the antigen source. Scand J Gastroenterol. 2003;38:50-54. [PubMed] [DOI] [Full Text] |
| 21. | Peracchi M, Trovato C, Longhi M, Gasparin M, Conte D, Tarantino C, Prati D, Bardella MT. Tissue transglutaminase antibodies in patients with end-stage heart failure. Am J Gastroenterol. 2002;97:2850-2854. [PubMed] [DOI] [Full Text] |
| 22. | Olerup O, Aldener A, Fogdell A. HLA-DQB1 and -DQA1 typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours. Tissue Antigens. 1993;41:119-134. [PubMed] [DOI] [Full Text] |
| 23. | Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29-36. [PubMed] [DOI] [Full Text] |
| 24. | Rätsch IM, Catassi C. Coeliac disease: a potentially treatable health problem of Saharawi refugee children. Bull World Health Organ. 2001;79:541-545. [PubMed] |
| 25. | Vivas S, Ruiz de Morales JM, Martinez J, González MC, Martín S, Martín J, Cechini C, Olcoz JL. Human recombinant anti-transglutaminase antibody testing is useful in the diagnosis of silent coeliac disease in a selected group of at-risk patients. Eur J Gastroenterol Hepatol. 2003;15:479-483. [PubMed] [DOI] [Full Text] |
| 26. | Dickey W, Hughes DF, McMillan SA. Disappearance of endomysial antibodies in treated celiac disease does not indicate histological recovery. Am J Gastroenterol. 2000;95:712-714. [PubMed] [DOI] [Full Text] |
| 27. | Kaukinen K, Sulkanen S, Mäki M, Collin P. IgA-class transglutaminase antibodies in evaluating the efficacy of gluten-free diet in coeliac disease. Eur J Gastroenterol Hepatol. 2002;14:311-315. [PubMed] [DOI] [Full Text] |
| 28. | Lionetti P, Favilli T, Chiaravalloti G, Ughi C, Maggiore G. Coeliac disease in Saharawi children in Algerian refugee camps. Lancet. 1999;353:1189-1190. [PubMed] [DOI] [Full Text] |
| 29. | Sblattero D, Berti I, Trevisiol C, Marzari R, Tommasini A, Bradbury A, Fasano A, Ventura A, Not T. Human recombinant tissue transglutaminase ELISA: an innovative diagnostic assay for celiac disease. Am J Gastroenterol. 2000;95:1253-1257. [PubMed] [DOI] [Full Text] |