Published online Jun 28, 2005. doi: 10.3748/wjg.v11.i24.3746
Revised: October 16, 2004
Accepted: December 1, 2004
Published online: June 28, 2005
AIM: To evaluate the preventive effect of Ginkgo biloba extract (GbE) on ethanol-induced gastric mucosal injuries in rats.
METHODS: Female Wistar albino rats were used for the studies. We randomly divided the rats for each study into five subgroups: normal control, experimental control, and three experimental groups. The gastric ulcers were induced by instilling 1 mL 50% ethanol into the stomach. We gave GbE 8.75, 17.5, 26.25 mg/kg intravenously to the experimental groups respectively 30 min prior to the ulcerative challenge. We removed the stomachs 45 min later. The gastric ulcers, gastric mucus and the content of non-protein sulfhydryl groups (NP-SH), malondialdehyde (MDA), c-Jun kinase (JNK) activity in gastric mucosa were evaluated. The amount of gastric juice and its acidity were also measured.
RESULTS: The findings of our study are as follows: (1) GbE pretreatment was found to provide a dose-dependent protection against the ethanol-induced gastric ulcers in rats; (2) the GbE pretreatment afforded a dose-dependent inhibition of ethanol-induced depletion of stomach wall mucus, NP-SH contents and increase in the lipid peroxidation (increase MDA) in gastric tissue; (3) gastric ulcer induced by ethanol produced an increase in JNK activity in gastric mucosa which also significantly inhibited by pretreatment with GbE; and (4) GbE alone had no inhibitory effect on gastric secretion in pylorus-ligated rats.
CONCLUSION: The finding of this study showed that GbE significantly inhibited the ethanol-induced gastric lesions in rats. We suggest that the preventive effect of GbE may be mediated through: (1) inhibition of lipid peroxidation; (2) preservation of gastric mucus and NP-SH; and (3) blockade of cell apoptosis.
-
Citation: Chen SH, Liang YC, Chao JC, Tsai LH, Chang CC, Wang CC, Pan S. Protective effects of
Ginkgo biloba extract on the ethanol-induced gastric ulcer in rats. World J Gastroenterol 2005; 11(24): 3746-3750 - URL: https://www.wjgnet.com/1007-9327/full/v11/i24/3746.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i24.3746
Ginkgo biloba, a member of the family Ginkgoaceae, was cultivated in China in the mid-1700s. Originating in the southeastern China some 200 million years ago, it has been living to an age of 1,000 years and it is the last remaining member of its order. Extract from the leaves of Ginkgo biloba (GbE) has been used as a traditional Chinese pharmacopeia for centuries in the treatment of asthma and bronchitis[1]. It is used medically today as a standardized preparation GbE (EGb 761) which contains 240 mg/g flavonoids (ginkgo-flavone glycosides) and 60 mg/g terpenoids (ginkgolides and bilobalides). Those are the most important active ingredients in the extract. The flavonoids act as free radical scavengers, especially for oxygen-derived free radicals, such as OH•, O2•-, RO•, and ROO•, and to neutralize ferryl ion-induced peroxidation[2,3]. The terpenoids is known as an antagonist of platelet-activating factor, which implicates in the processes of platelet aggregation and arterial thrombosis, acute inflam-mation, allergic reactions and cardiovascular insufficiency[4,5]. Recently GbE is used to improve cardiovascular circulation and to lessen cerebrovascular insufficiency in western countries clinically[6].
On the other hand, ethanol is well-known as a damaging agent to gastric mucosa in animal and clinical studies. At concentrations greater than 400 mL/L, it causes marked mucosal hyperemia, necrosis, edema and mucosal or submucosal hemorrage[7,8]. The formation of lesions may be mediated by oxygen-derived free radicals[9,10].
GbE is well-known as a strong free radical scavenger. The gastric mucosal injury after ethanol treatment may be mediated by free radicals. Only a few published studies showed the GbE protective effects on the ethanol-induced gastric mucosal lesion[11]. Thus, the present study was intended to evaluate the possible mechanisms of GbE protective effect on the ethanol-induced gastric ulcer in rats.
Female Wistar albino rats (Animal Center of National Taiwan University, Taipei, Taiwan) about 180-200 g were used for the study. We maintained the animals in air conditional room with 14/10 h light/dark cycles, fed them with regular chows, and allowed them free access to tap water. Food was deprived but free access to tap water was allowed 36 h before the experiment to ensure an empty stomach.
We purchased Ginkgo biloba extract (GbE, Cerenin® ampule, 3.5 mg GbE/mL) from DR. Willmar Schwabe Karlsruhe F.R.G. (Germany), and used normal saline as a vehicle.
The animals were divided into five subgroups for each study. GbE 8.75, 17.5, 26.25 mg/kg were given intravenously to the experimental groups respectively 30 min prior to the ulcerative challenge (50% ethanol, 1 mL) by orogastric gavage to the stomach of fasted rats. Each control and experimental group consisted of five rats.
We sacrificed animals under ether anesthesia 45 min after treatment with ethanol, and removed their stomachs which were opened along the greater curvature and examined for lesions developed in the glandular portion under dissecting microscope (×10) with a square grid. The numbers of ulcer lesions (U. No.) in the glandular portion of the stomach were noted. The ulcer area (mm2) were measured and expressed as the ulcer index (U.I.). We calculated the protective ratio (%) according to the following formula:
Preventive ratio (%) = (a-b)/a×100
a: the ulcer index of the control group
b: the ulcer index of the experimental group
The glandular stomach was removed and weighted. We transferred the glandular segments immediately to 0.1% Alcian blue solution in 0.16 mol/L sucrose solution with 0.05 mol/L sodium acetate to pH 5.0 and stained for 2 h at room temperature. After having rinsed with sucrose solution, we extracted the dye complexed with the gastric mucus with 0.05 mol/L magnesium chloride solutions. The aliquot of magnesium chloride solution (4 mL) was further extracted with equal volume of diethyl ether and centrifuged (3600 r/min, 10 min). Then, we calculated the quantity of Alcian blue extracted/g (net) of glandular tissue.
After the rats were killed, we opened the glandular stomachs and rinsed them in ice-cold saline, then stored them rapidly in a dry ice bath until analyzed. For the determination of NP-SH, the tissues were homogenized in ice-cold 50% (g/L) aqueous TCA and centrifuged. We determined the NP-SH by measuring the supernatants and 5,5’-dithiobis (2-nitrobenzoic acid) in phosphate buffer (pH 8.0). We read the absorbance (412 nm) 5 min after it is being incubated.
To determine the MDA, we incubated the supernatants with N-methyl-2-phenylindole and we read absorbance at 586 nm according to the manufacturer’s instructions (BIOXYTECH LPO-586 kits, OXIS International Inc., Portland, USA).
We fasted animals for 36 h. The following procedures were carried out under ether anesthesia. We gave GbE intravenously 15 min before pylorus ligation, and collected gastric juice 3 h after pylorus ligation. After its volume was measured and expressed in mEq/L, the gastric juice was centrifuged at 3000 r/min for 10 min. We also determined the total acidity of gastric juice by titrating it with 0.01 mol/L NaOH to pH 7.0.
Gastric mucosa was homogenized in Gold lysis buffer (40 mmol/L Tris-NaOH pH 7.5, 500 mmol/L NaCl, 0.1% NP-40, 6 mmol/L EDTA, 6 mmol/L EGTA, 10 mmol/L β-glycerophosphate, 10 mmol/L NaF, 10 mmol/L PNPP, 300 μmol/L sodium orthovanadate, 1 mmol/L benzamidine, 2 μmol/L PMSF, 10 mg/mL aprotinin, 1 μg/mL leupeptin, and 1 mmol/L DTT) and centrifuged. We collected the supernatant as total tissue lysate. Equal amounts of total tissue lysate (50 μg) were resolved by SDS-polyacrylamide gel electrophoresis (PAGE), and transferred onto Immobilon-P membrane (Millipore, Bedford, MA) as described previously[12]. We also incubated the membrane with an anti-JNK1 antiserum (Transduction Laboratories, Lexington, KY). The membranes were subsequently probed with anti-mouse IgG antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology) and visualized using enhanced chemiluminescence’s kits (ECL, Amersham). For kinase assay, we immunoprecipitated equal amounts of total tissue lysate (400 μg) with JNK1 specific antibody and protein A/G-PLUS agarose for 15 h at 4 °C. Kinase assay was carried out in 45 μL of kinase buffer (40 mmol/L Tris-NaOH pH 7.5, 500 mmol/L NaCl, 0.1% NP-40, 6 mmol/L EDTA, 6 mmol/L EGTA, 10 mmol/L β-glycerophosphate, 10 mmol/L NaF, 10 mmol/L PNPP, 300 μmol/L sodium orthovanadate, 1 mmol/L benzamidine, 2 μmol/L PMSF, 10 μg/mL aprotinin, 1 μg/mL leupeptin, and 1 mmol/L DTT) containing 5 μmol/L cold ATP, 10 μCi [γ-32P] ATP (5 000 Ci/mmoL, Amersham), and 1 μg GST-c-Jun fusion protein (Santa Cruz Biotechnology) as substrate, and incubated for 20 min at 25 °C. We mixed each sample with 8 μL of 5×Laemmli’s loading buffer to stop the reaction, heated for 10 min at 100 °C, and subjected to 8% SDS-PAGE. The gels were dried, visualized by autoradiography[12].
Results were expressed as mean±SE for each experiment. We analyzed the data with Student’s t-test. If a P value was less than 0.05, the differences were considered statistical1y significant.
Effect of GbE on ethanol-induced gastric mucosal damage
Table 1 shows all the rats that received 1 mL 50% ethanol administration had induced gastric mucosal damages. GbE (8.75-26.25 mg/kg, intravenously) suppressed the ethanol-induced gastric lesions including ulcer number (U. No.) and the ulcer index (U.I.) in a dose-dependent manner. As shown in Table 1, normal saline did not have any protective effect on gastric mucosal damage when given in the volume of 0.5-1.5 mL to rats intravenously. The maximal effect was obtained when GbE (26.25 mg/kg) was given 30 min prior to ethanol induction, resulting in a preventive ratio of 68.64% (P<0.05).
| Treatment and dose | n | Mucosal lesions² | Preventive ratio (%) | |
| U. Number | U.I. (mm2) | |||
| Control (NS) | 5 | 0 | 0 | |
| NS + 50% EtOH (1 mL/rat) | 5 | 10±4 | 20.31±4.23 | |
| GbE (8.75 mg/kg)+50% EtOH | 5 | 8±3 | 15.25±3.21 | 24.91 |
| GbE (17.5 mg/kg)+50% EtOH | 5 | 3±3a | 8.83±3.59a | 56.52 |
| GbE (26.25 mg/kg)+50% EtOH | 5 | 2±2a | 6.37±4.22a | 68.64 |
There was a significant decrease in the gastric mucus after treatment with 1 mL 50% ethanol. As shown in Table 2, pretreatment of GbE (8.75-26.25 mg/kg) significantly protected the decline in the gastric mucus levels which were induced by ethanol.
| Treatment and dose | n | Gastric wall mucus (mg Alcian blue/g wet tissue, mean±SE) |
| Control (NS) | 5 | 442.21±28.36 |
| NS+50% EtOH (1 mL/rat) | 5 | 356.87±30.67a |
| GbE (8.75 mg/kg)+50% EtOH | 5 | 348.87±32.37 |
| GbE (17.5 mg/kg)+50% EtOH | 5 | 395.27±25.91 |
| GbE (26.25 mg/kg)+50% EtOH | 5 | 410.82±29.16c |
Ethanol (50%, 1 mL) treatment significantly reduced the NP-SH concentration in the gastric mucosa as compared with control in rats. As shown in Table 3, GbE pretreatment significantly prevented the decrease in NP-SH concentrations in the dose of 8.75-26.25 mg/kg. However, during the GbE treatment alone there was no change in the concentration of NP-SH in gastric mucosa. Moreover, pretreatment of GbE significantly prevented lipid peroxidation induced by ethanol, the MDA concentrations were 209.27±10.48 or 118.82±8.26 in the rats that were treated with ethanol alone or combined with GbE, respectively (Table 4).
| Treatment and dose | n | NP-SH concentrations (mg/100 mg /wet tissue, mean±SE) |
| Control (NS) | 5 | 12.21±1.03 |
| NS+50% EtOH (1 mL/rat) | 5 | 2.82±1.32a |
| GbE (8.75 mg/kg)+50% EtOH | 5 | 5.03±1.25c |
| GbE (17.5 mg/kg)+50% EtOH | 5 | 5.66±1.82c |
| GbE (26.25 mg/kg)+50% EtOH | 5 | 11.32±2.01c |
| Treatment and dose | n | Malondialdehyde concentration (nmol/g wet tissue, mean±SE) |
| Control (NS) | 5 | 102.54±15.32 |
| NS+50% ethanol (1 mL/rat) | 5 | 209.27±10.48a |
| GbE (8.75 mg/kg)+50% EtOH | 5 | 212.32±9.83 |
| GbE (17.5 mg/kg)+50% EtOH | 5 | 125.48±11.39c |
| GbE (26.25 mg/kg)+50% EtOH | 5 | 118.82±8.26c |
GbE was intravenously administrated at a dose of 8.75-26.25 mg/kg 15 min before pylorus ligation. Control rats received the same volume of saline (0.5-1.5 mL). As shown in Table 5, GbE could not significantly decrease the gastric acid secretion and only slightly inhibited the gastric secretion and total acidity even at a dose of 26.25 mg/kg.
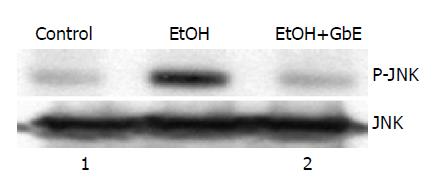
We examined c-Jun kinase (JNK) activity by using an immunocomplex kinase assay to explore whether the JNK signaling pathway is activated within gastric mucosa in response to ethanol, since JNK activation plays an important role in the induction of cell apoptosis. As shown in Figure 1, JNK activity was obviously increased after treatment with ethanol (top). However, GbE (26.25 mg/kg) significantly suppressed the induction of JNK activity. Western blot analysis revealed that this JNK activation was not caused by the enhanced expression of JNK protein (bottom).
In the past two decades, the protective factors of gastric mucosa were well evaluated. In 1979, Boyd et al[13], found that the gastric mucosa contains high concentrations of reduced glutathione, the major component of the endogenous NP-SH pool. In 1981, Szabo et al[3], showed the protective role of NP-SH in noxious-induced gastric injury. NP-SH pool might involve in scavenging oxygen-derived free radicals[14,15] and influence the production and character of mucus[16,17].
Mucus, which continuously coats over the gastric mucosa, is well known as a “mucous barrier” to prevent the injury of luminal acid, bacteria and noxious agents injuries[18,19]. Mucus might implicate in scavenging oxygen-derived free radicals[20,21]. Mucus glycoproteins and lipids bound to mucin might involve in the antiradical process[22,23].
On the other hand, previous reports offered that ethanol-induced gastric mucosal lesions may be attributed to the possible mechanisms: (1) increase oxygen-derived free radicals[9,10,24,25], (2) decrease the concentration of NP-SH contents in gastric mucosa[3,15], (3) direct damage to the mucin layer or mucin synthesis[26], and (4) causing gastric cell’s apoptosis[27-29].
GbE can remove free radicals especially oxygen-derived free radicals such as OH•, O2•-, RO•, and ROO•, and neutralize ferryl ion-induced peroxidation. GbE can improve the cardiovascular and cerebrovascular diseases e.g., Alzh-eimer’s disease, cerebral insufficiency and depression[6,30]. Furthermore, GbE has anti-inflammatory effect by decreasing the production of active oxygen and nitrogen species[31,32]. Recent reports showed that GbE can improve duodenal ulcer healing and reverses CCl4-induced liver fibrosis.
The results of the present study showed that pretreatment with GbE not only kept the mucus integrity (Table 2) but also inhibited ethanol-induced depletion in the NP-SH concentrations (Table 3) and MDA production in gastric mucosa (Table 4). All those findings in our study suggest that GbE has its role of being a free radical scavenger, decreases the lipid peroxidation, and blocks the loss of mucus, NP-SH, resulting in having protective effect on ethanol-induced mucosal injury.
Apoptosis has been implicated in causing ethanol-induced gastric mucosal injury[27-29]. Previous studies indicated that JNK kinase activity was elevated during the process of apoptosis and blocking of JNK activity was able to prevent cell apoptosis. The data from this study showed that ethanol significantly increased the JNK kinase activity, resulting in cell apoptosis and gastric mucosal damage. As shown in Figure 1, blocking of JNK activity may contribute to GbE’s gastric mucosal protection.
The finding of this study further showed that GbE did not significantly decrease the gastric acid secretion (Table 5). Our finding here is similar to the report by Wang et al in 2000[11].
In conclusion, the data of this study suggest that GbE can inhibit ethanol-induced gastric lesions in rats. The possible mechanisms of GbE’s antiulcer benefit may be due to its oxygen radicals scavenging by inhibition of lipid peroxidation, preventing the loss of gastric mucus and NP-SH, and blockade of ethanol-induced apoptosis. Further studies are warranted to evaluate GbE at the pharmacological effective dosage before any consideration for clinical trials.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Kleijnen J, Knipschild P. Ginkgo biloba. Lancet. 1992;340:1136-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 327] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 2. | Pincemail J, Dupuis M, Nasr C, Hans P, Haag-Berrurier M, Anton R, Deby C. Superoxide anion scavenging effect and superoxide dismutase activity of Ginkgo biloba extract. Experientia. 1989;45:708-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 114] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Gardès-Albert M, Ferradini C, Sekaki A, Droy-Lefaix MT. Oxygen-centered free radicals and their interactions with EGb 761 or CP 202. Advances in Ginkgo biloba extract research: Ginkgo biloba extract (EGb 761) as a free-radical scavenger. New York Elsevier Science 1993; 1-11. |
| 4. | Akiba S, Kawauchi T, Oka T, Hashizume T, Sato T. Inhibitory effect of the leaf extract of Ginkgo biloba L. on oxidative stress-induced platelet aggregation. Biochem Mol Biol Int. 1998;46:1243-1248. [PubMed] |
| 5. | Akisü M, Kültürsay N, Coker I, Hüseyinov A. Platelet-activating factor is an important mediator in hypoxic ischemic brain injury in the newborn rat. Flunarizine and Ginkgo biloba extract reduce PAF concentration in the brain. Biol Neonate. 1998;74:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Kleijnen J, Knipschild P. Ginkgo biloba for cerebral insufficiency. Br J Clin Pharmacol. 1992;34:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 187] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Oates PJ, Hakkinen JP. Studies on the mechanism of ethanol-induced gastric damage in rats. Gastroenterology. 1988;94:10-21. [PubMed] |
| 8. | Szabo S, Goldberg I. Experimental pathogenesis: drugs and chemical lesions in the gastric mucosa. Scand J Gastroenterol Suppl. 1990;174:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Pihan G, Regillo C, Szabo S. Free radicals and lipid peroxidation in ethanol- or aspirin-induced gastric mucosal injury. Dig Dis Sci. 1987;32:1395-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 178] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Szelenyi I, Brune K. Possible role of oxygen free radicals in ethanol-induced gastric mucosal damage in rats. Dig Dis Sci. 1988;33:865-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Wang Q, Zhao WZ, Ma CG. Protective effects of Ginkgo biloba extract on gastric mucosa. Acta Pharmacol Sin. 2000;21:1153-1156. [PubMed] |
| 12. | Liang YC, Huang YT, Tsai SH, Lin-Shiau SY, Chen CF, Lin JK. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis. 1999;20:1945-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 385] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 13. | Body SC, Sasame HA, Body MR. High concentrations of glutathione in glandular stomach: possible implications for carcinogenesis. Science. 1979;205:1010-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Videla LA, Valenzuela A. Alcohol ingestion, liver glutathione and lipoperoxidation: metabolic interrelations and pathological implications. Life Sci. 1982;31:2395-2407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 163] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Li T, Zhang XJ. Involvement of sulfhydryls in the protective mechanism of gastric mucosa. ShengLi XueBao. 1990;42:571-577. [PubMed] |
| 16. | Allen A, Cunliffe WJ, Pearson JP, Sellers LA, Ward R. Studies on gastrointestinal mucus. Scand J Gastroenterol Suppl. 1984;93:101-113. [PubMed] |
| 17. | Salim AS. Sulphydryl-containing agents and the prevention of duodenal ulcer relapse. Pharmacology. 1993;46:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Garner A, Flemström G, Allen A, Heylings JR, McQueen S. Gastric mucosal protective mechanisms: roles of epithelial bicarbonate and mucus secretions. Scand J Gastroenterol Suppl. 1984;101:79-86. [PubMed] |
| 19. | Farré AJ, Colombo M, Alvarez I, Glavin GB. Some novel 5-hydroxytryptamine1A (5-HT1A) receptor agonists reduce gastric acid and pepsin secretion, reduce experimental gastric mucosal injury and enhance gastric mucus in rats. J Pharmacol Exp Ther. 1995;272:832-837. [PubMed] |
| 20. | Grisham MB, Von Ritter C, Smith BF, Lamont JT, Granger DN. Interaction between oxygen radicals and gastric mucin. Am J Physiol. 1987;253:G93-G96. [PubMed] |
| 21. | Cross CE, Halliwell B, Allen A. Antioxidant protection: a function of tracheobronchial and gastrointestinal mucus. Lancet. 1984;1:1328-1330. [PubMed] |
| 22. | Gong DH, Turner B, Bhaskar KR, Lamont JT. Lipid binding to gastric mucin: protective effect against oxygen radicals. Am J Physiol. 1990;259:G681-G686. [PubMed] |
| 23. | Hiraishi H, Terano A, Ota S, Mutoh H, Sugimoto T, Harada T, Razandi M, Ivey KJ. Role for mucous glycoprotein in protecting cultured rat gastric mucosal cells against toxic oxygen metabolites. J Lab Clin Med. 1993;121:570-578. [PubMed] |
| 24. | Terano A, Hiraishi H, Ota S, Shiga J, Sugimoto T. Role of superoxide and hydroxyl radicals in rat gastric mucosal injury induced by ethanol. Gastroenterol Jpn. 1989;24:488-493. [PubMed] |
| 25. | Mutoh H, Hiraishi H, Ota S, Ivey KJ, Terano A, Sugimoto T. Role of oxygen radicals in ethanol-induced damage to cultured gastric mucosal cells. Am J Physiol. 1990;258:G603-G609. [PubMed] |
| 26. | Slomiany A, Morita M, Sano S, Piotrowski J, Skrodzka D, Slomiany BL. Effect of ethanol on gastric mucus glycoprotein synthesis, translocation, transport, glycosylation, and secretion. Alcohol Clin Exp Res. 1997;21:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Piotrowski J, Piotrowski E, Skrodzka D, Slomiany A, Slomiany BL. Gastric mucosal apoptosis induced by ethanol: effect of antiulcer agents. Biochem Mol Biol Int. 1997;42:247-254. [PubMed] |
| 28. | Mizushima T, Tsutsumi S, Rokutan K, Tsuchiya T. Suppression of ethanol-induced apoptotic DNA fragmentation by geranylgeranylacetone in cultured guinea pig gastric mucosal cells. Dig Dis Sci. 1999;44:510-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Hoshino T, Takano T, Tsutsumi S, Tomisato W, Tsuchiya T, Mizushima T. Effects of prostaglandin E2 on gastric irritant-induced apoptosis. Dig Dis Sci. 2002;47:2370-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun. 1994;201:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 581] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 31. | Kim HK, Son KH, Chang HW, Kang SS, Kim HP. Inhibition of rat adjuvant-induced arthritis by ginkgetin, a biflavone from ginkgo biloba leaves. Planta Med. 1999;65:465-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Yoshikawa T, Naito Y, Kondo M. Ginkgo biloba leaf extract: review of biological actions and clinical applications. Antioxid Redox Signal. 1999;1:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 4.2] [Reference Citation Analysis (0)] |