Published online Jun 28, 2005. doi: 10.3748/wjg.v11.i24.3729
Revised: April 25, 2004
Accepted: May 9, 2004
Published online: June 28, 2005
AIM: To investigate the clinicopathologic characteristics, immunophenotype and TCR gene rearrangements of hepatosplenic T-cell lymphoma in eight Chinese patients.
METHODS: Eight Chinese patients with hepatosplenic γδ T-cell lymphomas were studied. Hematoxylin-eosin-stained slides and clinical histories were reviewed. We also carried out immunohistochemical staining for CD3, CD4, CD8, CD20, CD43, CD56, CD79a, UCHL-1, and TCR γδ. Rearrangements of TCR gamma and delta chain genes were also studied.
RESULTS: The spleens were enlarged and the cut surfaces were homogeneous and red-purple in color without identifiable gross lesions or enlarged hilar lymph nodes. Histologically, lymphoma cells infiltrated the cords of Billroth and often packed the sinuses. Liver biopsy showed lymphoma cell infiltrations in the sinusoids, and three cases showed involvements of the portal tracts. Immunohistochemically lymphoma cells were positive for CD3, CD43, and CD56 in all cases. Four of eight cases were positive for CD8, and all cases were negative for CD4 (6/6). Monoclonal rearrangements of TCR γ gene were demonstrated by PCR analysis in five out of the eight cases. TCR δ gene rearrangements were detected in six out of the eight cases, which demonstrated single bands on PAGE gel, and the amplification products in two cases were confirmed by sequencing.
CONCLUSION: The clinicopathology of hepatosplenic γδ T-cell lymphoma in Chinese patients is similar to what was previously reported except that the splenomegaly is not so massive, and CD8 is positive.
- Citation: Wei SZ, Liu TH, Wang DT, Cao JL, Luo YF, Liang ZY. Hepatosplenic γδ T-cell lymphoma. World J Gastroenterol 2005; 11(24): 3729-3734
- URL: https://www.wjgnet.com/1007-9327/full/v11/i24/3729.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i24.3729
Hepatosplenic γδ T-cell lymphoma is a distinct clinicopathologic entity, and now recognized in the revised European-American lymphoma and World Health Organization classifications. It has uniform clinicopathologic characteristics: predominant occurrence in young male adults, hepatosplenomegaly presentation without lymphadenopathy, and peculiar sinusoidal pattern of infiltration of the spleen, liver, and bone marrow by monomorphic small to medium-sized tumor cells of TCR γδ immunophenotype[1].
There were few reports on hepatosplenic γδ T-cell lymphoma in Chinese patients in the literature. In this paper, we analyzed the clinicopathologic characteristics, immunophenotype and TCR gene rearrangements of hepatosplenic T-cell lymphoma in eight Chinese patients.
Eight cases of hepatosplenic γδ T-cell lymphoma diagnosed between 2000 and 2003 were found from the files of the Department of Pathology of Peking Union Medical College Hospital (PUMCH). Spleen and liver biopsies were obtained from seven of the eight cases. Splenectomy was performed on case 3 in another hospital, and the patient was admitted to our hospital for further treatment who finally died. Accessory spleen was found in autopsy. The jurkat cell line (T-cell leukemia) was used as a positive control for the detection of TCR γ gene rearrangements.
Pathological specimens were fixed in formalin. Hematoxylin-eosin-stained slides were prepared from paraffin blocks according to the standard protocol. Immunohistochemical staining was performed using DAKO EnVision and DAB peroxidase detection systems (Dako, Glostrup, Denmark). The antibodies to CD3, CD4, CD8, CD20, CD79a, CD43, CD56, and UCHL-1 were purchased from DAKO (Dako, Glostrup, Denmark) and TCR γδ antibody from Endogen (Woburn, MA, USA). Normal human spleen tissue (ruptured spleen of a young male adult caused by car accident) was used as a positive control for TCR γδ immunostaining, and target retrieval solution (pH 9.0) (Dako, Glostrup, Denmark) was used for the best demonstration.
Three to five 10 μm formalin-fixed and paraffin-embedded tissue sections were prepared for each sample. After being dewaxed with xylene and ethanol, tissue pellet was digested by proteinase K. Jurkat cells (3×106) were collected and cell pellet was also digested by proteinase K. Each DNA sample was verified for its suitability for PCR amplification by amplifying β-actin.
PCR of TCR δ gene rearrangements was performed with the primers Jδ1 and Vδ1b that amplified a fragment of about 120 bp[2].
Rearranged TCR γ genes was amplified using a method described by Signoretti et al[3]. PCR was carried out using the primers VG1, VG2, VG9, JG12-a, and JGP12-a which described by Muche et al[4]. T-cell leukemia cell line Jurkat was used as a positive control for TCR γ gene rearrangements. Ten microliters of the reaction products was analyzed by electrophoresis on 10% polyacrylamide gels, stained by ethidium bromide and viewed under UV light.
All the amplifications were carried out in a Hermocycle (HYBAID LIMITED, Middlesex, UK). Each sample was amplified at least three separate times to confirm the reproducibility of the results.
Because there was no positive control for TCR δ gene amplification, PCR products of TCR δ gene from cases 6 and 8, which were confirmed to have single target bands, were cut out and purified using a QIAEX gel extraction kit (QIAGEN Inc, Valencia, CA, USA). The purified products were then subjected to direct sequencing by an ABI377 sequencer (Perkin-Elmer Applied Biosystems, Foster City, CA, USA).
Careful measures were taken to avoid contamination during all steps of the analysis.
The clinical features are summarized in Table 1.
| CaseNumber | Sex | Age (yr) | Clinical symptoms | ALT <40(U/L) | ALP 27-107 (U/L) | g-GT 10-50 (U/L) | LDH 97-270 (U/L) | Platelet count 100-350 ×109/L | Hemoglobin >110 (G/L) |
| 1 | M | 12 | Fever, weight loss, hepatosplenomegaly, died 4 mo after diagnosis | 199 | 239 | NE | 899 | 104 | 79 |
| 2 | M | 22 | Fever, hepatosplenomegaly | NE | NE | NE | NE | NE | NE |
| 3 | M | 25 | Fever, weight loss, hepatosplenomegaly, jaundice, died 1 mo after diagnosis | 775 | 563 | 214 | 775 | 88 | 103 |
| 4 | F | 28 | Fever, hepatosplenomegaly abdominal pain | 55 | 246 | 66 | Normal | 108 | 100 |
| 5 | M | 30 | Fever, weight loss, hepatosplenomegaly | 71 | 506 | 169 | 358 | 49 | 95 |
| 6 | F | 30 | Fever, weight loss, hepatosplenomegaly, abdominal pain | 155 | 500 | 115 | 518 | 74 | 91 |
| 7 | M | 36 | Fever, weight loss, hepatosplenomegaly, jaundice; died 4 mo after diagnosis | 409 | 350 | 121 | 389 | 90 | 143 |
| 8 | M | 52 | Fever, weight loss, hepatosplenomegaly, abdominal pain, jaundice | Normal | 159 | 82 | Normal | 64 | 107 |
Our study consisted of 6 men and 2 women with a median age of 29 years (ranging from 12 to 52 years). The ages of all the patients were under 36 years except one case whose age was 52 years.
The patients were admitted to PUMC Hospital for fever and hepatosplenomegaly. Other features included weight loss and abdominal pain, and jaundice. Liver tests of most cases were abnormal, with elevated levels of serum γ-glutamyl-transpeptidase (γ-GT), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) activities. Five cases showed elevated serum lactate dehydrogenase (LDH) levels.
As shown in Table 1, anemia was found in six cases (hemoglobin levels ranging from 79 to 107 G/L). Thrombo-cytopenia was present in five patients (platelet counts ranging from 49 to 90/L).
All patients had splenomegaly and hepatomegaly at the time of diagnosis. Physical examination revealed no enlarged superficial lymph node. CT scan disclosed no mediastinal or retroperitoneal lymphadenopathy except case 5, in whom mediastinal lymphadenopathy was discovered.
All the patients had no significant past history, including receiving long-term immunosuppressive therapy.
Four cases were followed-up. Three of them died within 1-6 mo after operation, and 1 case continued to receive treatment a year-and-a-half after the diagnosis.
Pathological feathers are summarized in Table 2. The spleens were enlarged, weighing 445-1155 g and the mean splenic weight was 675 g. The cut surfaces were homogeneous and red-purple in color without identifiable gross lesions or hilar lymph node enlargement.
| Case No. | Spleen weight (g) | Gross appearance (cut surface) of spleen | Microscopical findings | Lymphoid involvement | Bone marrow involvement |
| 1 | 910 | Homogeneous red-purple, no nodule, soft | Lymphoma cells infiltrate liver sinus, splenic sinusoids; liver portal tract, liver fatty degeneration | NO | NO |
| 2 | 800 | Homogeneous dull-red, firm | Lymphoma cells infiltrate liver sinus, splenic sinusoids | NE | NE |
| 3 | Part of accessory spleen | Homogeneous gray-red, firm | Lymphoma cells infiltrate liver sinus, splenic sinusoids; liver fatty degeneration | NO | NO |
| 4 | 445 (body weight 45 kg) | Homogeneous gray-red, no nodule | Lymphoma cells infiltrate liver sinus, splenic sinusoids | NO | NO |
| 5 | 1155 | Homogeneous gray-red, firm | Lymphoma cells infiltrate liver sinus, splenic sinusoids; liver fatty degeneration | Mediastinal lymphadenopathy | NO |
| 6 | 800 | Homogeneous gray-red, soft | Lymphoma cells infiltrate liver sinus, splenic sinusoids; liver portal tract, liver fatty degeneration | NO | NO |
| 7 | 810 | Homogeneous dull-brown, no nodule | Lymphoma cells infiltrate liver sinus, splenic sinusoids; liver portal tract, liver fatty degeneration | NO | Suspected lymphoid cell |
| 8 | 480 (body weight 45 kg) | Homogeneous dull-red, soft | Lymphoma cells infiltrate liver sinus, splenic sinusoids | NO | Suspected lymphoid cell |
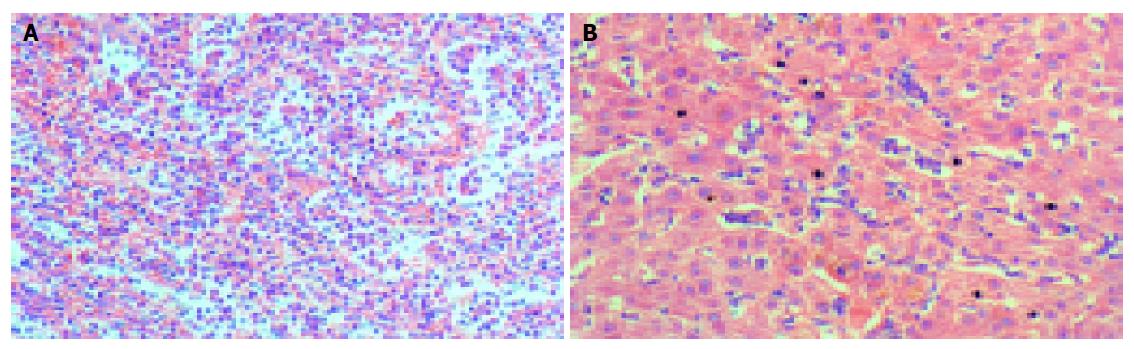
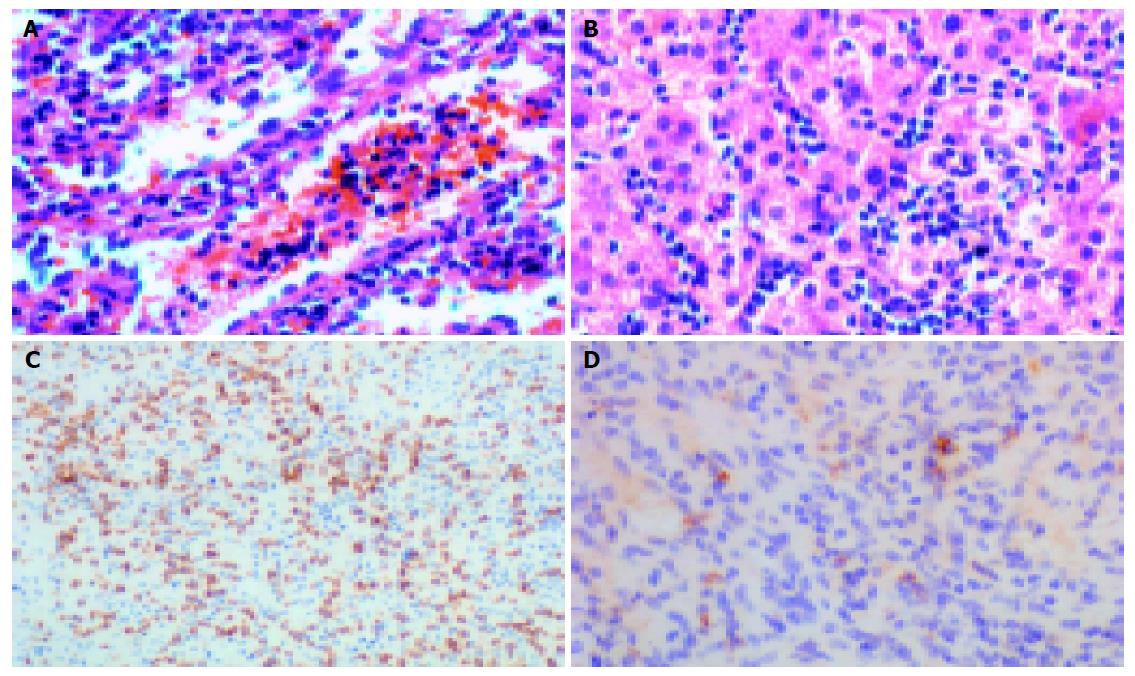
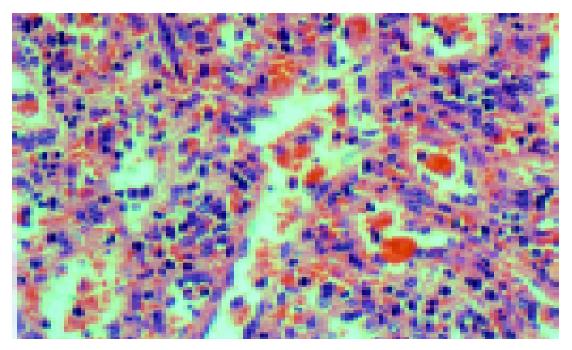
Histologically, there were marked expansion of the red pulp and atrophy of the white pulp in the spleens. Lymphoma cells infiltrated the cords of Billroth and often packed the sinuses (Figures 1 and 2A). Lymphoma cells were usually of small to medium sizes with round or oval nuclei showing slightly dispersed chromatin, and scant to moderate cytoplasm. Seven of eight spleens showed erythrophagocytosis more or less (Figure 3).
Liver biopsy was performed in all the patients except case 3, whose whole liver was available for examination. Lymphoma cells infiltrated and distended the liver sinusoids (Figures 1 and 2B), and three cases demonstrated involvement of portal tract. Liver biopsies of five cases exhibited mild to moderate fatty degeneration.
Bone marrow aspirate smears were performed in these cases. But only two cases demonstrated abnormal findings suggesting lymphoma infiltrates.
As shown in Table 3, neoplastic cells in all cases expressed T-cell associated markers, i.e., CD3 (Figure 2C), UCHL-1 and CD43, whereas B-cell associated markers including CD20 and CD79A were negative. Tumor cells in all the cases expressed CD56 antigen. Six cases were negative for CD4 (6/6), whereas four cases expressed CD8 (4/8). Positive TCR γδ immunostaining on routinely formalin-fixed and paraffin-embedded tissue sections was observed in Golgi region of lymphoma cells in five cases (5/7) (Figure 2D).
| CD20 | CD79a | CD3 | CD4 | CD8 | CD43 | UCHL-1 | CD56 | TCRγδ | TCR γ gene rearrangement | TCR δ gene rearrangement | |
| 1 | - | - | + | - | - | + | + | + | - | - | + |
| 2 | - | - | + | - | - | + | + | + | NE | + | + |
| 3 | - | - | + | NE | - | + | + | + | + | + | - |
| 4 | - | - | + | - | + | + | + | + | + | - | - |
| 5 | - | - | + | - | + | + | + | + | + | + | + |
| 6 | - | - | + | - | + | + | + | + | + | - | + |
| 7 | - | - | + | NE | + | + | + | + | + | + | + |
| 8 | - | - | + | - | - | + | + | + | - | + | + |
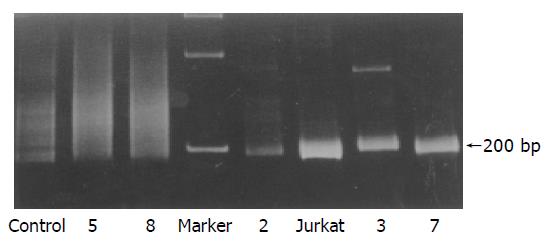
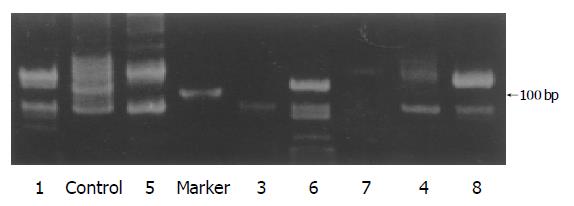
Monoclonal rearrangements of TCR γ gene were demonstrated by PCR analysis in five of the eight cases (Table 3), which were single fragments of about 200 bp (Figure 4).
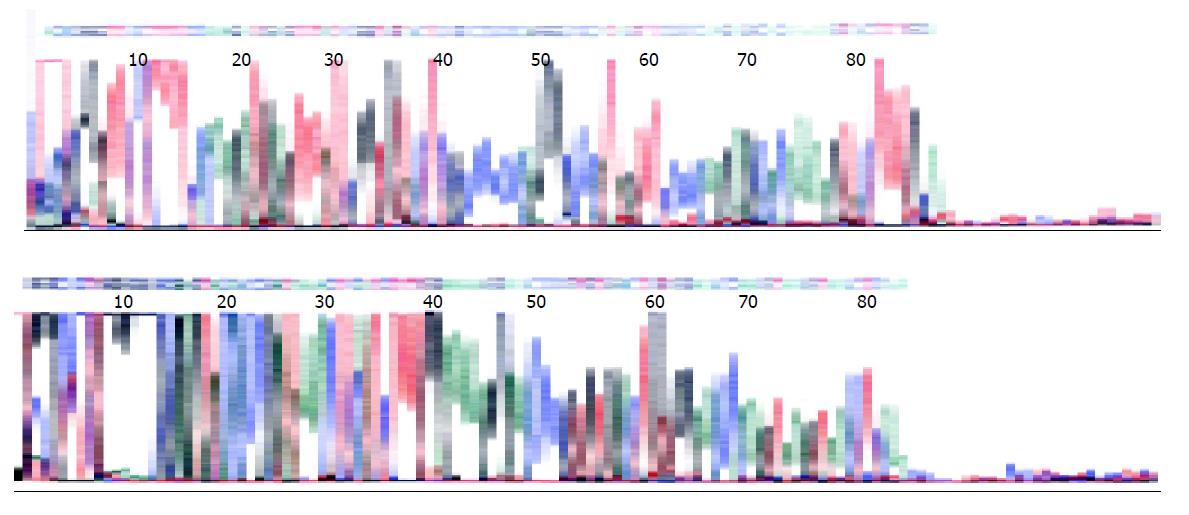
Complete TCR δ gene rearrangements were detected using primers Vδ1b and Jδ1 which amplified fragments of about 120 bp (Figure 5). Six out of eight cases demonstrated single bands on PAGE gel. The amplification products of TCR δ gene in two cases (cases 6 and 8) were also subjected to direct sequencing by an ABI377 sequencer (Perkin-Elmer Applied Biosystems, Foster City, CA, USA). The results were consistent with the rearranged TCR δ gene through the search of BLAST in PubMed (Figure 6).
In this study we analyzed eight hepatosplenic T-cell lymphomas in Chinese patients. The results confirmed a predilection for young male adults. Splenomegaly and hepatomegaly were present in all cases and coexisted lymphadenopathy was found in one case. The weights of spleens from cases 4 and 8 were 445 and 480 g, respectively. It seemed that the weights were lighter than those reported in the literature, but these two patients were thin, weighing 45 and 47 kg respectively, and their spleens were evidently enlarged, the lower edges were both at the level of umbilicus in physical examination.
The pathological findings were similar to those previously reported in the literature. Neoplastic cells were usually monomorphic small to medium-sized lymphocytes. Tumor cells were located preferentially in sinusoids of the liver, cords and sinuses of the splenic red pulp. However, besides sinusoidal infiltrates of the liver, portal tract involvements were also present in three cases in this series, and liver biopsies of five cases demonstrated mild to moderate fatty degeneration. According to Gaulard et al[1] and Belhadj et al[5], a mild portal and periportal lymphomatous infiltrate might also be observed but was not predominant[1,5]. However, Weidmann et al[6], indicated that marked or even exclusive portal infiltration could be present. Lymphomatous infiltration resulted in chronic liver injury, and most of our cases revealed abnormal liver function. Perfetto et al, described a case of hepatosplenic lymphoma whose clinical course mimicked acute hepatitis. It reminded us that hepatosplenic T-cell lymphoma should be included in the differential diagnosis of acute hepatic dysfunction in young patients who showed no evidence of viral, toxic, autoimmune or metabolic liver diseases.
In previous reports most of TCR γδ immunophenotypes were demonstrated on frozen sections and it was difficult to detect these antigens on routinely formalin-fixed and paraffin-embedded sections. In this study, we tried to demonstrated the TCR γδ immunostaining on paraffin-embedded sections using target retrieval solution (high pH) (Dako, Glostrup, Denmark) following Macon’s protocol[7]. TCR γδ immunohistochemistry on formalin-fixed and paraffin-embedded sections demonstrated Golgi region staining in five cases. The failure of the rest three cases may be due to the TCR γδ antigen denaturation caused by formalin fixation. Our results confirmed that TCR γδ immunostaining could be demonstrated on formalin-fixed and paraffin-embedded sections, although not all the cases revealed positive staining.
For detecting monoclonal TCR γ gene, Southern blot analysis for TCR γ chain gene rearrangements remains a reliable and reproducible technique with minimal false positive results. PCR technique is also a reliable and reproducible technique, but paraffin sample TCR γ PCR testing is less reliable than fresh or frozen tissue reported by most laboratories, and the risk for false negative results in paraffin-embedded tissues should be recognized[8]. In this study, five of the eight cases demonstrated monoclonal rearrangements of TCR γ gene.
TCR δ gene consists of at least six V δ gene segments. Over 95% of γδ T-cells express either Vδ1 or Vδ2 gene. Interestingly, normal γδ T lymphocytes in spleen, thymus, and intestinal epithelium predominantly express the Vδ1 gene, whereas the majority of γδ T-cells in peripheral blood, tonsils, and skin express the Vδ2 gene. Grzegorz et al demonstrated usage of Vδ1 gene and Vδ2 gene were found in hepatosplenic γδ T-cell lymphoma (10/11 cases) and in γδ subcutaneous panniculitis-like γδ T-cell lymphoma (4/4 cases), respectively.
In our study, Vδ1 and Jδ1 gene rearrangements were demonstrated in six of eight cases. The result further support hepatosplenic γδ T-cell lymphoma is preferentially derived from the Vδ1 subset of γδ T lymphocytes.
It seemed that the results of rearrangements of TCR γ and TCR δ genes were not accordant. There were two reasons for this discordance. First, in this study, we used formalin fixed and paraffin embedded samples to perform immunohistochemistry and to extract DNA for PCR amplification because of lack of fresh or frozen tissue. Archival formalin-fixed and paraffin-embedded samples could frequently lead to protein denaturation and DNA degradation. Second, we only employed the most commonly used primers to detect the rearrangements of TCR γ and TCR δ genes. Maybe there were uncommon gene rearrangements in the remaining cases.
In conclusion, the clinicopathology of hepatosplenic γδ T-cell lymphoma in Chinese patients is similar to what was previously reported in the literature.
Science Editor Wang XL Language Editor Elsevier HK
| 1. | Gaulard P, Belhadj K, Reyes F. Gammadelta T-cell lymphomas. Semin Hematol. 2003;40:233-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Przybylski GK, Wu H, Macon WR, Finan J, Leonard DG, Felgar RE, DiGiuseppe JA, Nowell PC, Swerdlow SH, Kadin ME. Hepatosplenic and subcutaneous panniculitis-like gamma/delta T cell lymphomas are derived from different Vdelta subsets of gamma/delta T lymphocytes. J Mol Diagn. 2000;2:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Signoretti S, Murphy M, Cangi MG, Puddu P, Kadin ME, Loda M. Detection of clonal T-cell receptor gamma gene rearrangements in paraffin-embedded tissue by polymerase chain reaction and nonradioactive single-strand conformational polymorphism analysis. Am J Pathol. 1999;154:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Muche JM, Lukowsky A, Asadullah K, Gellrich S, Sterry W. Demonstration of frequent occurrence of clonal T cells in the peripheral blood of patients with primary cutaneous T-cell lymphoma. Blood. 1997;90:1636-1642. [PubMed] |
| 5. | Belhadj K, Reyes F, Farcet JP, Tilly H, Bastard C, Angonin R, Deconinck E, Charlotte F, Leblond V, Labouyrie E. Hepatosplenic gammadelta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood. 2003;102:4261-4269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 300] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | Weidmann E. Hepatosplenic T cell lymphoma. A review on 45 cases since the first report describing the disease as a distinct lymphoma entity in 1990. Leukemia. 2000;14:991-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 162] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Macon WR, Salhany KE. T-cell subset analysis of peripheral T-cell lymphomas by paraffin section immunohistology and correlation of CD4/CD8 results with flow cytometry. Am J Clin Pathol. 1998;109:610-617. [PubMed] |
| 8. | Arber DA, Braziel RM, Bagg A, Bijwaard KE. Evaluation of T cell receptor testing in lymphoid neoplasms: results of a multicenter study of 29 extracted DNA and paraffin-embedded samples. J Mol Diagn. 2001;3:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |