Published online Jun 28, 2005. doi: 10.3748/wjg.v11.i24.3701
Revised: August 1, 2004
Accepted: August 25, 2004
Published online: June 28, 2005
AIM: To explore the alterations of intestinal mucosa morphology, and the effects of tumor necrosis factor α (TNFα) on enterocyte apoptosis in mice with fulminant hepatic failure (FHF).
METHODS: Liver damage was induced by lipopolysaccharide (LPS)/TNF-α in D-galactosamine (GalN) sensitized BALB/c mice. There were 40 mice in normal saline (NS)-treated group, 40 mice in LPS-treated group, 40 mice in GalN-treated group, 120 mice in GalN/ LPS-treated group and 120 mice in GalN/ TNFα-treated group. Each group was divided into five subgroups of eight mice each. Serum samples and liver, intestinal tissues were respectively obtained at 2, 6, 9, 12 and 24 h after administration. Anti-TNFα monoclonal antibody was injected intravenously into GalN/LPS-treated mice. Serum TNFα levels were determined by enzyme-linked immunosorbent assays (ELISA). Serum ALT levels were determined using an automatic analyzer. The intestinal tissues were studied under light microscope and electron microscope at 2, 6, 9, 12 and 24 h in mice with fulminant hepatic failure, respectively. Enterocyte apoptosis was determined by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) method. The expression of tumor necrosis factor receptor 1 (TNFR1) in intestinal tissue was tested by immunohistochemistry Envision Two Steps.
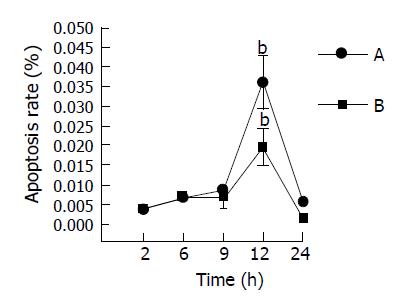
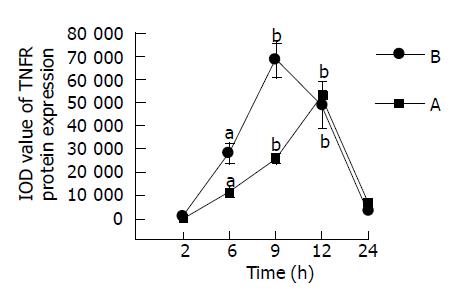
RESULTS: Gut mucosa was morphologically normal at all time points in all groups, but typical apoptotic cells could be seen in all experimental groups under electron micro-scope. Apoptosis rate of gut mucosal epithelial cells were significantly increased at 6, 9 and 12 h, peaked at 12 h in mice with fulminant hepatic failure. TNFα induced apoptosis of enterocytes in mice with FHF. The integrated OD (IOD) levels of TNFα receptor 1 protein expressed in the intestine of mice with GalN/LPS and GalN/ TNFα-induced FHF at 2, 6, 9, 12 and 24 h after GalN/LPS and GalN/TNFα administration were 169.54±52.62/905.79±111.84, 11350.67±2133.26/28160.37±4601.67, 25781.00±2277.75/122352.30±49412.40, 5241.53±3007.24/49157.93±9804.88, 7086.13±1031.15/3283.45±127.67, respectively, compared with those in control groups (with NS, LPS and GalN administration, respectively). IOD level of TNFR1 changed significantly at 6, 9 and 12 h after GalN/LPS and GalN/TNFα administration. The expression of TNFR1 protein was significantly higher at 9 h after GalN/LPS and GalN/TNFα administration than that in control groups. Protein expression of TNFR1 was positively correlated with enterocyte apoptosis.
CONCLUSION: TNFα can induce apoptosis of enterocytes in mice with FHF. Anti-TNFα IgG can inhibit this role.
- Citation: Song HL, Lu S, Liu P. Tumor necrosis factor-alpha induces apoptosis of enterocytes in mice with fulminant hepatic failure. World J Gastroenterol 2005; 11(24): 3701-3709
- URL: https://www.wjgnet.com/1007-9327/full/v11/i24/3701.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i24.3701
Tumor necrosis factor α (TNFα) is a key cytokine with a broad-spectrum of physiopathological effect, and secreted mainly by monokaryons and macrophages. Besides participating in humoral and cell immunity, TNFα also plays an important role in many diseases such as severe hepatitis, septic shock and inflammatory bowel disease[1-5].
Patients with severe hepatitis are at high risk for serious complications such as spontaneous bacterial peritonitis (SBP), which is an important cause of death in patients with severe hepatitis[6-8]. SBP is related with the changes of intestinal permeability, bacterial translocation, etc.[9-12], as well as the production of inflammatory mediators such as TNF, IFN-γ and IL[13,14]. However, whether there is a relationship between acute liver necrosis and barrier function of intestinal mucosa injury is still unknown.
No study has reported the important role of intestinal function in patients with fulminant hepatic failure (FHF) complicated by SBP. Additional studies are needed to clarify the degree of mucosal damage and gut barrier dysfunction induced by FHF, and if possible, to explore the potential mechanisms. Therefore, we performed a series of studies on intestines of mice with FHF. The purpose of this study was to explore the alterations of intestinal mucosa morphology, and the effects of TNFα on apoptosis of enterocytes in mice with FHF.
Six-week-old male BALB/c mice (provided by Laboratory Animal Center in China Medical University, China) were housed at a constant room temperature and humidity with free access to food and water, and subjected to a 12-h light/dark cycle. Food was withdrawn overnight before experiments. Mice with fulminant hepatic failure (FHF) were given an intraperitoneal injection of D-galactosamine (GalN) (800 mg/kg body weight, Sigma), followed by lipopolysaccharide (LPS) (100 μg/kg body weight, Sigma). For preparation of mice with GalN/TNFα-treated induced fulminant hepatic failure mice were given an intraperitoneal injection of GalN (800 mg/kg body weight, Sigma), followed by TNFα (10 μg/kg body weight, Sigma). In control groups, mice were given an intraperitoneal injection of GalN (800 mg/kg body weight, Sigma), LPS (100 mg/kg body weight, Sigma), NS (equal volume GalN and LPS, body weight), respectively. There were 40 mice in NS-treated group, 40 mice in LPS-treated group, 40 mice in GalN-treated group, 120 mice in GalN/ LPS-treated group and 120 mice in GalN/TNFα-treated group. Each group was divided into five subgroups of eight mice each. Serum samples and liver, intestinal tissues were respectively obtained at 2, 6, 9, 12 and 24 h. Anti-TNFα monoclonal antibody (100 μg/mouse, Fitzgerald) was injected intravenously into GalN/LPS-treated mice 1 h before GalN/LPS administration. Serum samples and liver, intestinal tissues were respectively obtained at 9 h (5 mice) after administration of GalN/ LPS.
Serum levels of alanine transaminase (ALT) were deter-mined, liver and intestinal tissues were fixed for histopathologic analysis. Intestinal tissues were fixed for electron and light microscopy. Liver and intestinal specimens were taken and stained with hematoxylineosin. Morphological change was observed under transmission electron microscope.
Serum alanine transaminase (ALT) levels were determined using an automatic analyzer (HITACHI 7250; Hitachi, Japan). Serum TNFα (TNFα mouse EIA kit; Genzyme, Cambridge, MA) was determined by enzyme-linked immunosorbent assays (ELISA) according to the manufacturer’s protocol.
Samples for electron microscopy were fixed in phosphate-buffered glutaraldehyde (2.5%) and osmium tetroxide (1%). Mucosa was dehydrated in acetone solution. The tissue was embedded in an epoxy resin. One micrometer thick section was made and stained with toluidine blue. Six hundred angstrom-thin sections were made from a selected area of tissue and stained with lead citrate and uranyl acetate. Ultrastructures of mucosa were observed under a transmission electron microscope (H-600; Hitachi, Japan).
After liver and intestine tissues were fixed in 10% phosphate buffered formalin and embedded in paraffin, 5-μm thick serial sections were stained with hematoxylineosin for histopathologic analysis. Apoptotic enterocytes were detected by the in situ terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) method according to the test instructions of kit.
Sections were indirectly immunolabeled with an ABC kit according to the manufacturer’s instructions. Sections were deparaffined and diluted in normal goat serum for 20 min. Following incubation with polyclonal antibody (rabbit anti-mouse TNFR1 diluted in phosphate-buffered saline (PBS) 1:100; Sigma) applied for 12 h in a humidified chamber at 4 °C. These sections were then rinsed thrice for 10 min in PBS, and the secondary antibody (goat anti-rabbit IgG), diluted 1:100 in PBS was applied for 2 h at room temperature. Sections were rinsed in PBS as before and then rinsed in distilled water. Fresh peroxidase reaction mixture containing equal amounts of 0.02% hydrogen peroxide in H2O and 0.1% diaminobenzidine in PBS were prepared. Sections were mounted on Uvinert mountant (BDH, UK). Positively stained cells were expressed as integrated A value.
We chose three fields of vision in microscopy at random, and analyzed the positive rate of apoptotic cells and TNFR1 protein expression using ‘multi-system color/RGB monitor’ computer image processing system. Accounting formula was positively cells stained (rate) = the area of brown cells/total area×100%.
Software SPSS 11.0 was used in statistical analysis. Each parameter was expressed as mean±SE, and compared using one-way ANOVA, followed by Tukey test. P<0.05 was considered statistically significant.
Administration of lipopolysaccharide (LPS, 10 μg/kg body weight/ TNFα 100 μg/mouse) and D-galactosamine (GalN, 800 mg/kg body weight) respectively induced acute liver necrosis. Nine hours after GalN/LPS or GalN/TNFα administration, the mortality of mice reached 60% (72/120) and 60.83% (73/120). However, the mortality of mice with acute liver necrosis induced by GalN/LPS did not increase after 12 h. The total mortality was 66.6% (80/120) and 73.3% (87/120).
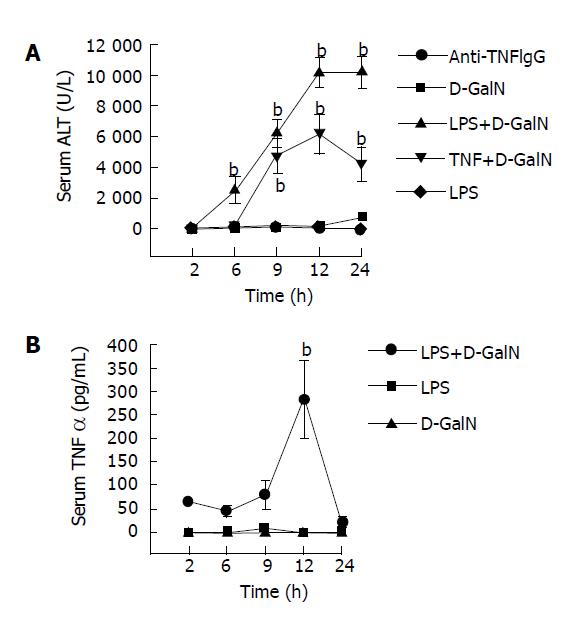
Serum ALT levels in LPS, GalN, and NS-treated mice were almost normal at different time points. But serum TNFα levels in GalN/LPS and GalN/TNFα-treated mice began to increase at 6 h after GalN/LPS administration (2513.15± 874.18 U/L) and (204.1±82.05 U/L), 9 h (6235.45±912.43 U/L) and (4774.82±1117.95 U/L), and reached a maximal level at 12 h (10215.75±967.71 U/L) and (6177.84± 1280.96 U/L). It began to decrease after 12 h (10251.94 ±104581 U/L).
Anti-TNFα IgG significantly reduced serum ALT levels in mice with fulminant hepatic failure at 9 h after GalN/LPS administration (6235.45±912.43 U/L vs 257.1± 83.17 U/L, P<0.01) (Figure 1A).
Serum TNFα levels in LPS, GalN and NS-treated mice were almost normal at different time points. But serum TNFα levels in GalN/LPS-treated mice began to increase and reached a maximal level at 12 h (201.12±81.73 ng/L) after treatment with LPS, GalN, NS and GalN/LPS. It began to decrease at 24 h (22.37±12.03 ng/L) (Figure 1B).
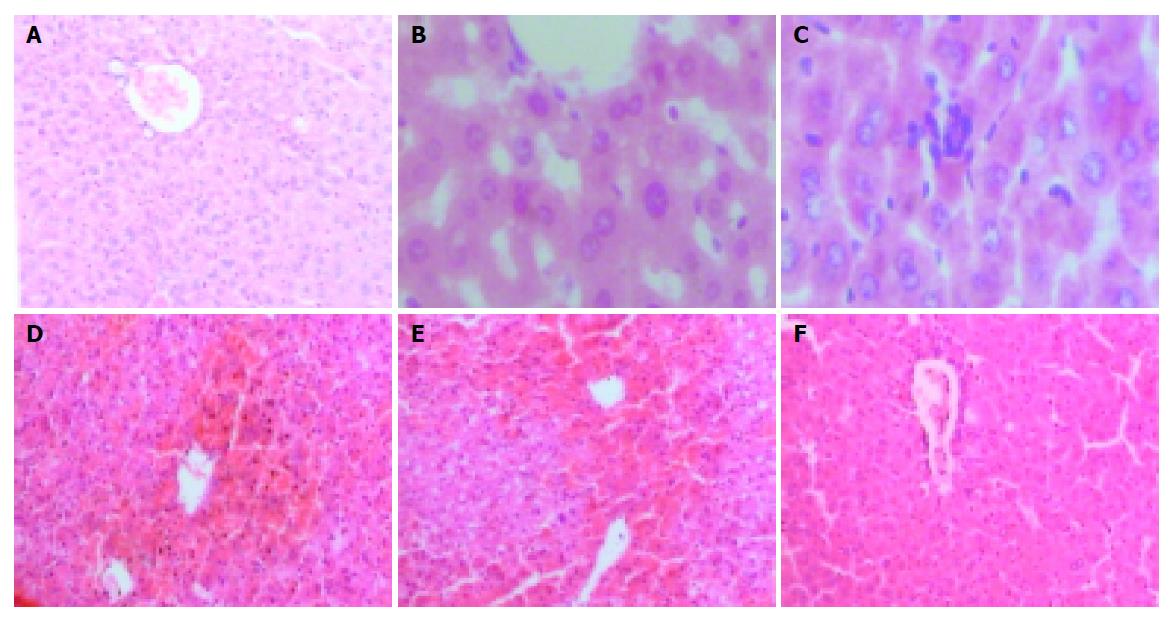
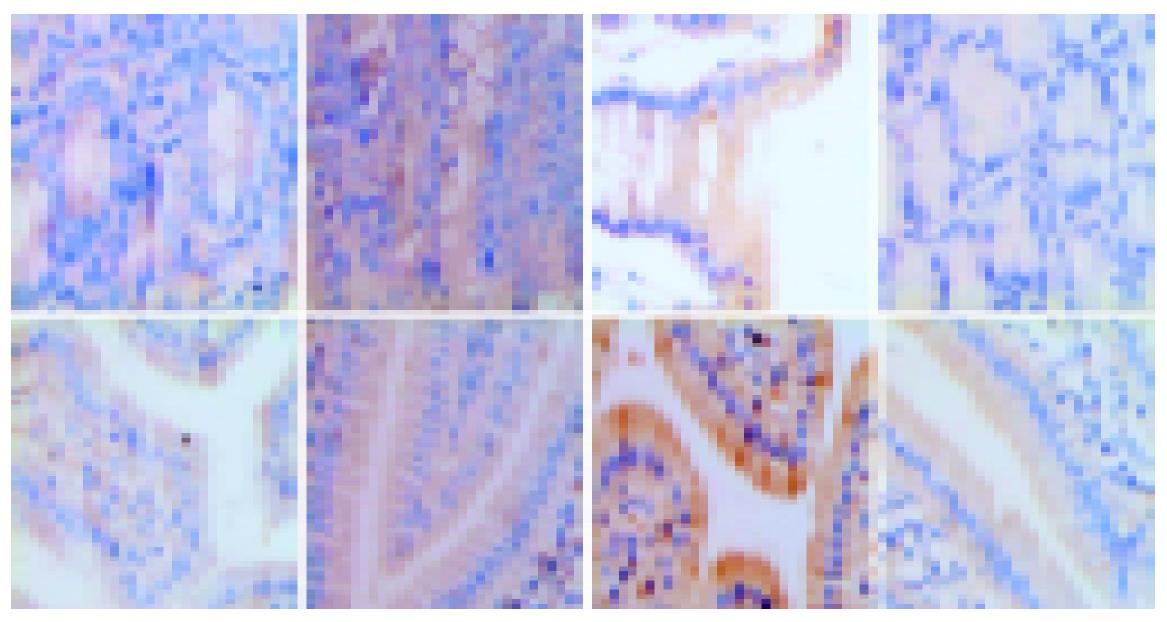
Nine hours after injecting GalN/LPS and GalN/TNFα, liver tissues from most mice with acute liver necrosis/FHF presented severe hemorrhage and hepatic necrosis, but acidophil degeneration or formation of apoptotic bodies was found in some residual hepatocytes. The hepatic cord disappeared and the structure of liver leaflet deranged. Fatty degeneration and swelling were observed in LPS-treated group. However, degeneration or spotty necrosis of hepatocytes, congestion, acidophil degeneration and dilatation of hepatic sinusoids were found in GalN-treated group. Nine hours after anti-TNFα monoclonal antibody was injected intravenously into GalN/LPS-treated mice, liver tissues in most mice did not present severe hemorrhage and hepatic necrosis, but some residual hepatocytes appeared as spotty and focal necrosis. Cloudy swelling of hepatocytes was seen in central veins.
The degrees of liver injury in mice with acute liver necrosis were increased remarkably as compared with control group (Figure 2).
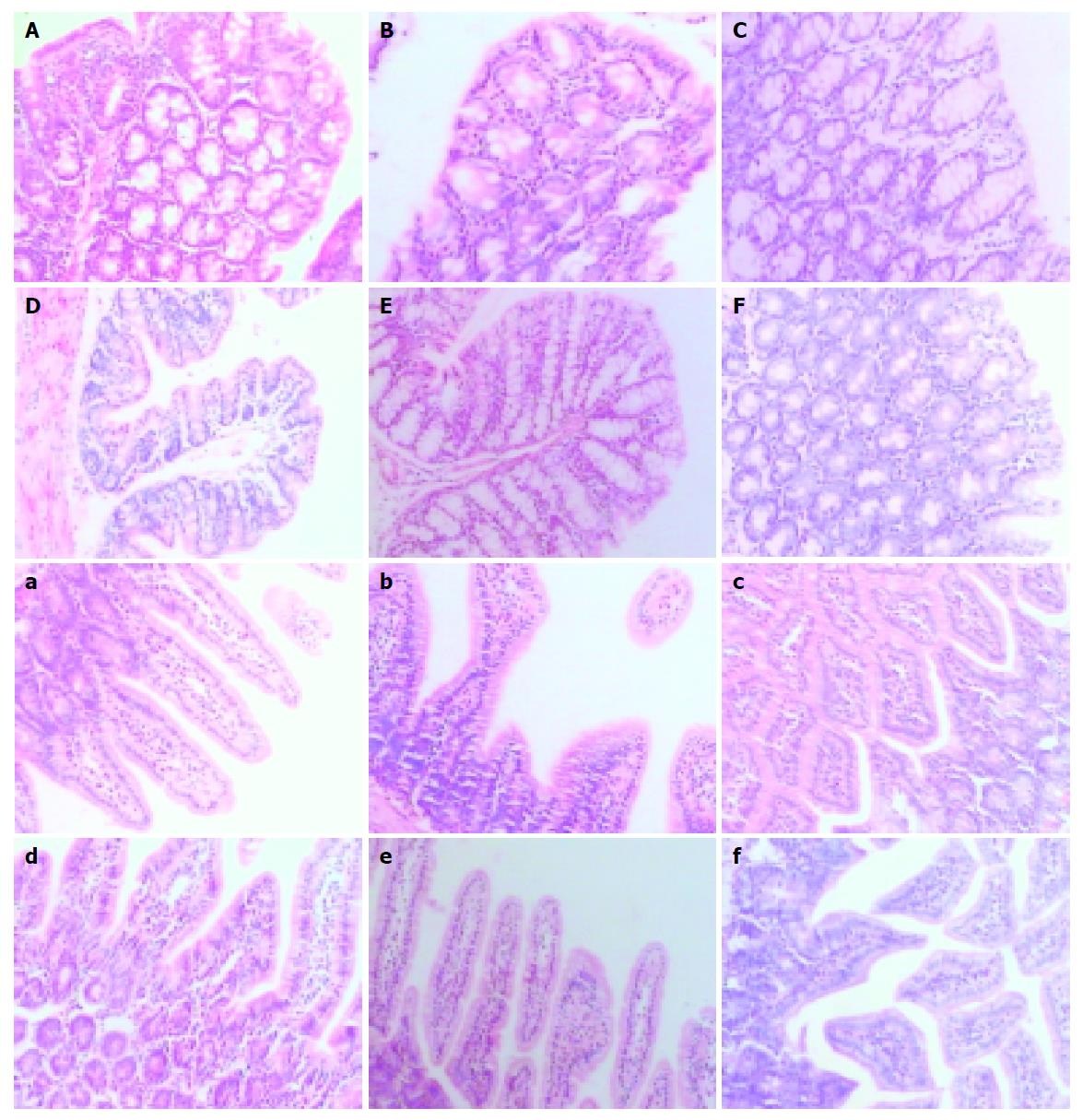
Intestinal samples at different time points were taken for hematoxylineosin (HE) staining. Pathomorphology of intestinal mucosa was not remarkably altered in mice with acute liver necrosis compared with that in control groups. Epithelial cells shed normally from the top of villi and villi were arranged 9 h after treatment (Figure 3).
TEM analysis of apoptosis in colon and small intestinal epithelia of acute liver necrosis and control was performed.
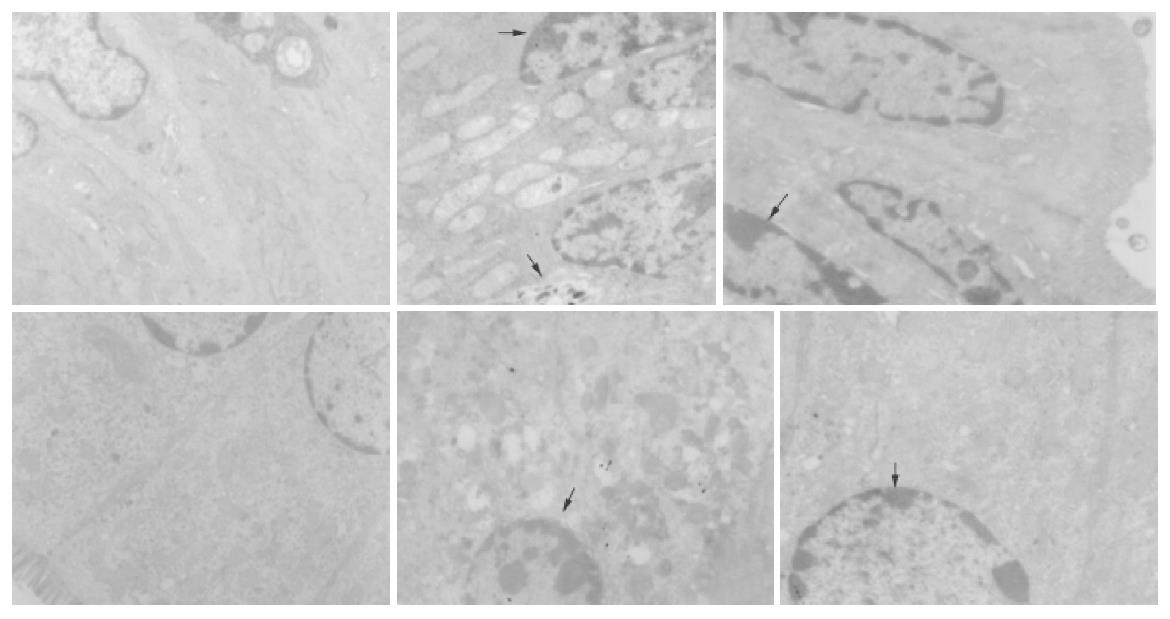
In transmission electron microscopy (TEM), well-developed structure of intestinal epithelial apoptosis was clearly observed in small intestine and colon of experiment groups. There were significant differences in the appearance and number of apoptosis cells between liver necrosis and control groups.
In GalN/LPS-treated group, the nuclei of epithelial cells were irregular, but their membranes were still intact. Obvious ultrastructural alterations were found, including reduction of matrix of mitochondria and disruption of their cristae, ruptured, distorted and sparse microvilli, apoptosis bodies in cytoplasms, disrupted and wider tight junction between epithelial cells, swollen and degenerated vascular endothelial cells. The ultrastructure of intestinal epithelium was almost normal. In anti-TNFα IgG-treated group, the ultrastructural alterations were small (Figure 4).
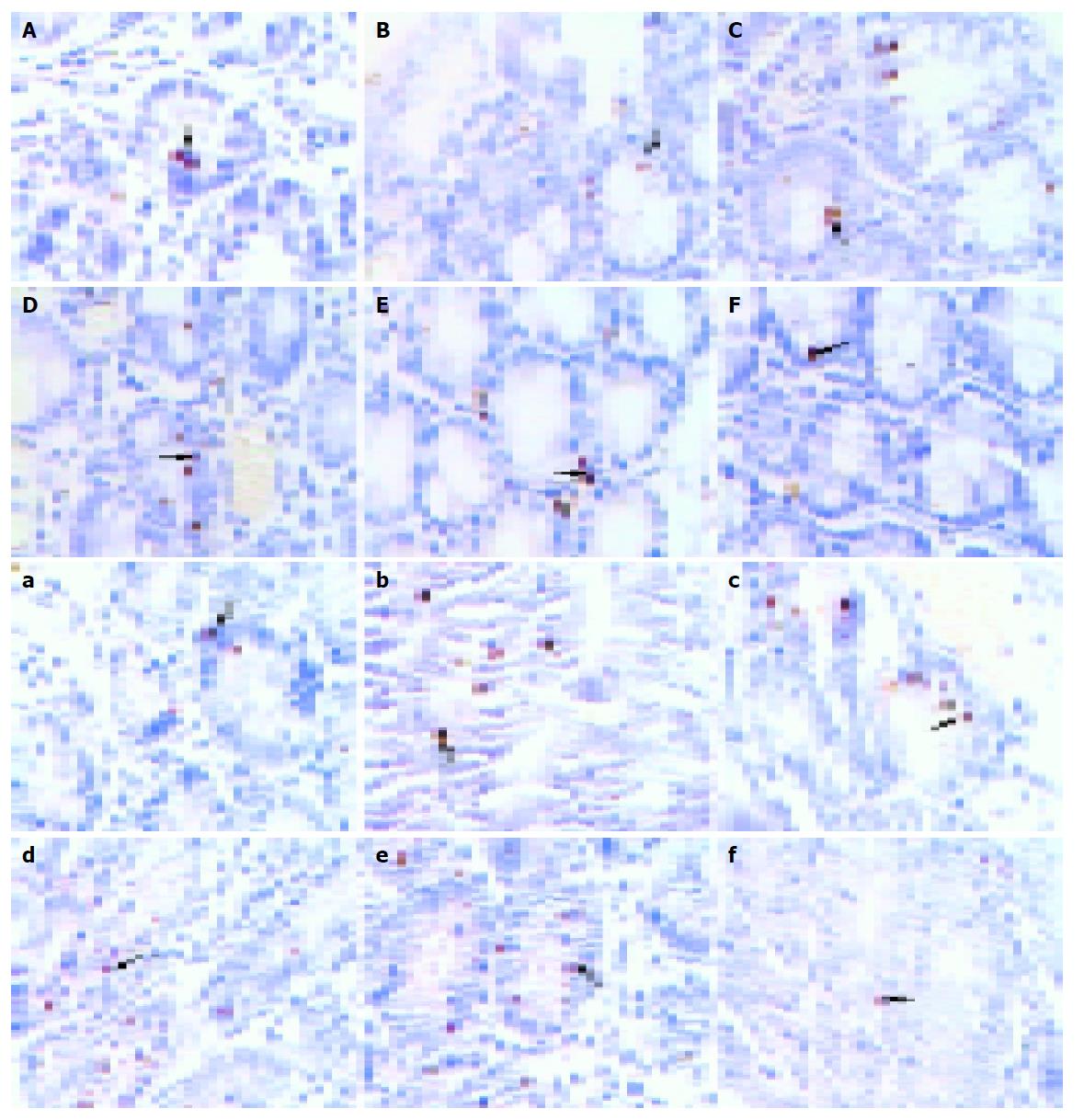
In this study, we examined the effects of TNFα on apoptosis in the intestine of NS, GalN, LPS, anti-TNFαIgG, GalN/LPS and GalN/TNFα-treated mice. Few TUNEL-positive cells were found in the intestine specimens of NS, GalN, LPS-treated mice 2 h after treatment. There were a few TUNEL-positive cells in GalN/LPS and GalN/TNFα-treated mice 2 h after treatment (Figure 5). A small number of apoptotic enterocytes were seen in the intestines of GalN/LPS and GalN/TNFα-treated mice 6 and 9 h after treatment. A large number of TUNEL-positive enterocytes were seen in the intestine of GalN/LPS and GalN/TNFα-treated mice 12 h after treatment. In NS, LPS, GalN, and anti-TNFα IgG-treated groups, a few TUNEL-positive cells were found in the intestine specimens 9 h after treatment (Figure 6). These findings indicate that anti-TNFα antibody could inhibit enterocyte apoptosis in GalN/LPS-treated mice.
The changes of TNFR1 protein expression are shown in Figure 7. At 9 h, in LPS and DalN-treated groups, the expression was less than that in GalN/LPS-treated groups. But in anti-TNFα IgG-treated group, the expression was decreasing.
We analyzed the TNF related to TNFα-induced apoptotic pathways by means of computer image processing system assays. The IOD levels of TNFR1 protein expression in the intestine of mice with FHF at 2, 6, 9, 12, and 24 h after treatment with GalN/LPS and GalN/TNFα, were compared with those of control mice (Figure 8). However, IOD value of TNFR1 changed significantly at 6, 9, and 12 h after treatment with GalN/LPS and GalN/TNFα. The expression of TNFR1 proteins was significantly higher at 9 h after GalN/LPS and GalN/TNFα treatment than other control groups. Anti-TNFα IgG inhabited the expression of TNFR1 proteins in mice with acute liver necrosis. There was a slight difference in IOD level of TNFR1 between large intestine and small intestine tissues (the peak time point was at 9 h in small intestine, but at 12 h in large intestine).
The pathogenesis of severe hepatitis complicated by SBP still remains obscure. Intestinal tract is not only an important organ of digestion and absorption of nutrients, but also prevents bacterial penetration[15]. The function of the intestinal tract in monitoring and preventing intruders is called the gut barrier. A variety of specific and nonspecific mechanisms are in operation to establish the host barrier[16,17]. Recent study showed that acute hepatic failure results in intestinal endotoxemia (IETM), and increases intestinal mucosal permeability[18]. The intestinal mucosal barrier is composed of mucosal fluid, microvilli, epithelium mucosae cell tight junction and special structure[19]. Maintaining the stability of epithelial mucosa depends on the balance between proliferation and apoptosis of epithelial cells[20]. Once some changes occur in components, it can result in dysfunction of the barrier.
Many scholars have discovered that the level of serum TNFα is obviously increased in patients with severe hepatitis[21]. We made the animal model of FHF induced by GalN/LPS or GalN/TNFα. The clinical manifestation, biochemistry analysis and liver histopathology are coincident with FHF[22,23]. We clearly demonstrated that anti-TNFα antibody could prevent the necrosis of liver in mice with fulminant hepatic failure induced by GalN/LPS.
Recent studies showed that TNFα binds to the soluble tumor necrosis factor receptor, (sTNFR), sTNFR 1(55P) and sTNFR 2(75P) are expressed in intestinal epithelial cells[24]. Members of TNF and TNF receptor families play important roles in inducing apoptosis. TNF induces apoptosis of enterocytes[25,26] and increases the permeability[27-29]. TNFα causes cell apoptosis by activating caspases-3 in cells, but TNFα-induced apoptosis of enterocytes in mice is mediated by TNF receptor 1[30]. The empirical study on TNF-induced apoptosis of enterocytes demonstrated that TNFα-induced apoptosis requires activation of ICE caspase family whereas complete inhibition of the caspase cascade leads to necrotic cell death[31]. At the same time, TNFα has cytotoxic action, which directly causes edema and destroys tissues. Apoptosis accounts for about half of TNFα-induced permeability [32]. Corredor[33] found that a physiological TNFα level (1 μg/L) enhances migration through TNFR2, whereas a pathological TNFα level (100 μg/L) inhibits wound healing through TNFR1. TNFα is a prominent cytokine in the pathogenesis of severe hepatitis, yet its effects on intestinal epithelium in mice with severe hepatitis remain poorly understood.
In our studies we observed apoptosis of large and small intestinal tissues at different time points in the mice model of FHF. The result of electron microscopy confirmed the occurrence of apoptosis in intestine of mice with FHF. But the apoptosis enterocytes in large and small intestine dramatically decreased in anti-TNFα IgG group. Therefore we suggest that TNFα can induce apoptosis of enterocytes in mice with FHF, and anti-TNFα IgG can inhibit its action. Apoptosis is not only a physiologic phenomenon of death, but also involves many processes of disease. In common, in a 24 h period, there may be 960-1200 apoptotic bodies per villus, which are approximately equal to the villus influx to maintain the physiological function of intestinal tract barrier[34].
Regulation of the cells in adult tissues is determined by the balance between cell production and loss. In the gastrointestinal tract, where there are well-defined zones of proliferation and migration of both epithelial cells and associated fibroblasts, cell loss occurs by shedding into the gut lumen. Taking the absolute number of apoptotic bodies into consideration, the rapid clearance and the size of small intestinal villi and colonic crypts indicate that the cell loss in normal murine intestine can largely be explained by the observed apoptosis. Though being inconspicuous in histological material, apoptosis probably accounts for the bulk of cell loss in the gut and is a central feature of the regulation of cell number in intestinal tissues.
The biological action of TNFα is brought into full play through two distinct cell surface receptors, TNFR1 (55 ku) and TNFR2 (75 ku), and both of them could combine with TNFα and TNFβ. TNFR2 only plays a role in signal transmission[35]. TNF binding to its receptor enters cells by receptor mediating endocytosis, and induces cytolysis and cytotoxic action[36,37]. In contrast to the rapid onset of apoptosis seen after bacterial infection of mouse monocyte-macrophage cell lines, the commitment of human intestinal epithelial cell lines to undergo apoptosis is delayed for at least 6 h after bacterial infection, and the ensuing phenotypic expression of apoptosis is delayed for 12-18 h after bacterial entry. TNFα and nitric oxide produced as components of the intestinal epithelial cell proinflammatory program in the early period after bacterial invasion, play an important role in the later induction and regulation of the epithelial cell apoptotic program[38]. Apoptosis in response to bacterial infection might function to delete infected and damaged epithelial cells and restore epithelial cell growth regulation and epithelial integrity that are altered during the course of enteric infection. In our study, immunohistochemistry analysis indicated that the expression of TNFR1 protein was significantly higher at 9 h after GalN/LPS and GalN/TNFα administration, than that in control groups. Protein expression of TNFR1 is positively correlated with enterocyte apoptosis. The time of TNFα-induced apoptosis of enterocytes was coincident with that of hepatocytes in FHF[22]. Therefore, TNFα could induce apoptosis of enterocytes in mice with GalN/LPS-induced fulminant hepatic failure. This apoptosis is mediated by a positive apoptotic signal leading to activation of the caspase (ICE family of proteases) cascade via TNFR1-associated death domain protein and additional apoptotic signals via receptor-interacting proteins.
In conclusion, apoptosis of enterocytes occurs with high serum level of TNFα in severe hepatitis complicated by SBP, and the regulation of intestinal cell apoptosis by TNFα seems to be mediated by TNFR1, and anti-TNFα IgG can inhibit intestinal cell apoptosis by reducing the expression of TNFR1.
| 1. | Leist M, Gantner F, Bohlinger I, Tiegs G, Germann PG, Wendel A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol. 1995;146:1220-1234. [PubMed] |
| 2. | Song HL, Lu S, Ma L, Li Y, Liu P. Effect of TNFα on tight junctions between the epithelial cells of the intestinal mucosa barrier. Shijie Huaren Xiaohua Zazhi. 2004;12:1303-1306. |
| 3. | Lu S, Song HL, Wang JY, Liu P. Blood brain barrier permeability in acute liver necrosis of mice. Shijie Huaren Xiaohua Zazhi. 2004;12:1346-1348. |
| 4. | Corredor J, Yan F, Shen CC, Tong W, John SK, Wilson G, Whitehead R, Polk DB. Tumor necrosis factor regulates intestinal epithelial cell migration by receptor-dependent mechanisms. Am J Physiol Cell Physiol. 2003;284:C953-C961. [PubMed] [DOI] [Full Text] |
| 5. | Kanzler S, Galle PR. Apoptosis and the liver. Semin Cancer Biol. 2000;10:173-184. [PubMed] [DOI] [Full Text] |
| 6. | Poddar U, Thapa BR, Prasad A, Sharma AK, Singh K. Natural history and risk factors in fulminant hepatic failure. Arch Dis Child. 2002;87:54-56. [PubMed] [DOI] [Full Text] |
| 7. | Thanopoulou AC, Koskinas JS, Hadziyannis SJ. Spontaneous bacterial peritonitis (SBP): clinical, laboratory, and prognostic features. A single-center experience. Eur J Intern Med. 2002;13:194-198. [PubMed] [DOI] [Full Text] |
| 8. | França AV, De Souza JB, Silva CM, Soares EC. Long-term prognosis of cirrhosis after spontaneous bacterial peritonitis treated with ceftriaxone. J Clin Gastroenterol. 2001;33:295-298. [PubMed] [DOI] [Full Text] |
| 9. | Such J, Francés R, Muñoz C, Zapater P, Casellas JA, Cifuentes A, Rodríguez-Valera F, Pascual S, Sola-Vera J, Carnicer F. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36:135-141. [PubMed] [DOI] [Full Text] |
| 10. | Chang CS, Yang SS, Kao CH, Yeh HZ, Chen GH. Small intestinal bacterial overgrowth versus antimicrobial capacity in patients with spontaneous bacterial peritonitis. Scand J Gastroenterol. 2001;36:92-96. [PubMed] [DOI] [Full Text] |
| 11. | Chiva M, Guarner C, Peralta C, Llovet T, Gómez G, Soriano G, Balanzó J. Intestinal mucosal oxidative damage and bacterial translocation in cirrhotic rats. Eur J Gastroenterol Hepatol. 2003;15:145-150. [PubMed] [DOI] [Full Text] |
| 12. | Solà R, Soriano G. Why do bacteria reach ascitic fluid? Eur J Gastroenterol Hepatol. 2002;14:351-354. [PubMed] [DOI] [Full Text] |
| 13. | Rodríguez-Ramos C, Galan F, Díaz F, Elvira J, Martín-Herrera L, Girón-González JA. Expression of proinflammatory cytokines and their inhibitors during the course of spontaneous bacterial peritonitis. Dig Dis Sci. 2001;46:1668-1676. [PubMed] [DOI] [Full Text] |
| 14. | Viallon A, Zeni F, Pouzet V, Lambert C, Quenet S, Aubert G, Guyomarch S, Tardy B, Bertrand JC. Serum and ascitic procalcitonin levels in cirrhotic patients with spontaneous bacterial peritonitis: diagnostic value and relationship to pro-inflammatory cytokines. Intensive Care Med. 2000;26:1082-1088. [PubMed] [DOI] [Full Text] |
| 15. | Wu BJ, Wang JD, Zhang YL. The progress of reseach in intestinal mucosa barrier. Shijie Huaren Xiaohua Zazhi. 2003;11:619-623. |
| 16. | Harari Y, Weisbrodt NW, Moody FG. Ileal mucosal response to bacterial toxin challenge. J Trauma. 2000;49:306-313. [PubMed] [DOI] [Full Text] |
| 17. | Kiyono H, Kweon MN, Hiroi T, Takahashi I. The mucosal immune system: from specialized immune defense to inflammation and allergy. Acta Odontol Scand. 2001;59:145-153. [PubMed] [DOI] [Full Text] |
| 18. | Duan ML, Wang YJ, Han DW. Studies on intestinal mucisa barrier injuries in acute hepatic failure. Zhongguo Bingli Shengli Zazhi. 1999;15:906-908. |
| 19. | Yue MX. Diagnosis and treatment in disfunction and failure of the gastrointestinal tract. Shijie Huaren Xiaohua Zazhi. 2002;10:3-6. |
| 20. | Potten CS. Epithelial cell growth and differentiation. II. Intestinal apoptosis. Am J Physiol. 1997;273:G253-G257. [PubMed] |
| 21. | Nakama T, Hirono S, Moriuchi A, Hasuike S, Nagata K, Hori T, Ido A, Hayashi K, Tsubouchi H. Etoposide prevents apoptosis in mouse liver with D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure resulting in reduction of lethality. Hepatology. 2001;33:1441-1450. [PubMed] [DOI] [Full Text] |
| 22. | Yu YS, Zang GQ, Jiang H, Le WL, Wang WZ. The expression of TRADD in liver tissues of mice with fulminant hepatic failure induced by TNFα. Ganzhang Zazhi. 2004;9:18-20. |
| 23. | Leist M, Gantner F, Bohlinger I, Germann PG, Tiegs G, Wendel A. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-alpha requires transcriptional arrest. J Immunol. 1994;153:1778-1788. [PubMed] |
| 24. | Gitter AH, Bendfeldt K, Schulzke JD, Fromm M. Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis. FASEB J. 2000;14:1749-1753. [PubMed] [DOI] [Full Text] |
| 25. | Kanzler S, Galle PR. Apoptosis and the liver. Semin Cancer Biol. 2000;10:173-184. |
| 26. | Pinkoski MJ, Droin NM, Green DR. Tumor necrosis factor alpha up-regulates non-lymphoid Fas-ligand following superantigen-induced peripheral lymphocyte activation. J Biol Chem. 2002;277:42380-42385. [PubMed] [DOI] [Full Text] |
| 27. | Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999;112:137-146. [PubMed] |
| 28. | Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell. 2002;13:3218-3234. [PubMed] [DOI] [Full Text] |
| 29. | Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367-G376. [PubMed] [DOI] [Full Text] |
| 30. | Piguet PF, Vesin C, Guo J, Donati Y, Barazzone C. TNF-induced enterocyte apoptosis in mice is mediated by the TNF receptor 1 and does not require p53. Eur J Immunol. 1998;28:3499-3505. [PubMed] [DOI] [Full Text] |
| 31. | Ruemmele FM, Dionne S, Levy E, Seidman EG. TNFalpha-induced IEC-6 cell apoptosis requires activation of ICE caspases whereas complete inhibition of the caspase cascade leads to necrotic cell death. Biochem Biophys Res Commun. 1999;260:159-166. [PubMed] [DOI] [Full Text] |
| 32. | Gitter AH, Bendfeldt K, Schulzke JD, Fromm M. Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis. FASEB J. 2000;14:1749-1753. |
| 33. | Corredor J, Yan F, Shen CC, Tong W, John SK, Wilson G, Whitehead R, Polk DB. Tumor necrosis factor regulates intestinal epithelial cell migration by receptor-dependent mechanisms. Am J Physiol Cell Physiol. 2003;284:C953-C961. |
| 34. | Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569-3577. [PubMed] |
| 35. | Barbara JA, Smith WB, Gamble JR, Van Ostade X, Vandenabeele P, Tavernier J, Fiers W, Vadas MA, Lopez AF. Dissociation of TNF-alpha cytotoxic and proinflammatory activities by p55 receptor- and p75 receptor-selective TNF-alpha mutants. EMBO J. 1994;13:843-850. [PubMed] |
| 36. | Loetscher H, Pan YC, Lahm HW, Gentz R, Brockhaus M, Tabuchi H, Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990;61:351-359. [PubMed] [DOI] [Full Text] |
| 37. | Schall TJ, Lewis M, Koller KJ, Lee A, Rice GC, Wong GH, Gatanaga T, Granger GA, Lentz R, Raab H. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990;61:361-370. [PubMed] [DOI] [Full Text] |
| 38. | Kim JM, Eckmann L, Savidge TC, Lowe DC, Witthöft T, Kagnoff MF. Apoptosis of human intestinal epithelial cells after bacterial invasion. J Clin Invest. 1998;102:1815-1823. [PubMed] [DOI] [Full Text] |