Published online Jun 28, 2005. doi: 10.3748/wjg.v11.i24.3686
Revised: February 8, 2004
Accepted: March 2, 2004
Published online: June 28, 2005
AIM: To evaluate the killing effect of double suicide gene mediated by adenovirus and regulated under kinase domain insert containing receptor (KDR) promoter on human umbilical vein endothelial cells.
METHODS: By PCR technology, human KDR promoter gene, Escherichia coli (E. coli) cytosine deaminase (CD) gene and the herpes simple virus-thymidine kinase (TK) gene were cloned. Plasmid pKDR-CDglyTK was constructed with them. Then, a recombinant adenoviral plasmid pAdKDR-CDglyTK was constructed in a “two-step transformation protocol”. The newly constructed plasmids were transfected to 293 packaging cells to grow adenoviruses, which were further propagated and purified. Human umbilical vein endothelial cells (HUVEC) were infected with a different multiplicity of infection (MOI) of resultant recombinant adenovirus, the infection rate was measured with the aid of (GFP) expression. Infected cells were cultured in culture media containing different concentrations of (GCV) and/or 5-(FC), and the killing effects were measured.
RESULTS: Recombinant adenoviruses AdKDR-CDglyTK were successfully constructed, and they infected HUVEC cells efficiently. Our data indicated that the infection rate was relevant to MOI of recombinant adenoviruses. HUVEC cells infected with AdKDR-CDglyTK were highly sensitive to the prodrugs, their survival rate correlated to both the concentration of the prodrugs and the MOI of recombinant adenoviruses. Our data also indicated that the two prodrugs used in combination were much more effective on killing transgeneic cells than GCV or 5-FC used alone.
CONCLUSION: Prodrug/KDR-CDglyTK system is effective on killing HUVEC cells, its killing effect correlates to the concentration of prodrugs and recombinant adenovirus’ MOI. Combined use of the two prodrugs confers better killing effects on transgeneic cells.
- Citation: Huang ZH, Yang WY, Cheng Q, Yu JL, Li Z, Tong ZY, Song HJ, Che XY. Kinase domain insert containing receptor promotor controlled suicide gene system kills human umbilical vein endothelial cells. World J Gastroenterol 2005; 11(24): 3686-3690
- URL: https://www.wjgnet.com/1007-9327/full/v11/i24/3686.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i24.3686
It has long been established that blood supply is required for a tumor to progress in excess of 1-2 mm3[1,2]. Anti-angiogenic therapy has been proved to be a rational approach in the treatment of solid tumors[3-8]. Gene therapy is a novel technology that might lead to improved treatments of some types of cancer[9]. Both approaches are promising in tumor therapy. But how about a combination of these two methods, using suicide genes to abrogate the tumor vasculature?
A suicide gene is a gene encoding an enzyme that converts nontoxic prodrugs into toxic forms. Researches indicated that apart from direct killing effects of a suicide gene, which kills its host cells only, its “bystander effects” could offer death to cells nearby. These characteristics allow a therapeutic application of suicide genes to tumors. TK gene is one of the most widely studied suicide genes[10], and CD enzyme gene is also widely studied[11-13]. But both of them have their shortcomings. The fusion genes of TK and CD are proposed to be new suicide genes with a better therapeutic action[14-17].
Human umbilical vein endothelial cells (HUVEC) are primary cells that can be used to investigate the mechanisms related to the role of endothelial cells[18]. KDR gene is strictly expressed only in vascular endothelial cells. The activity of the KDR promoters in endothelial cells is similar to that of the potent SV40 promoter/enhancer and this high level activity is specific to endothelial cells, while the activity of KDR promoters in other cell types is markedly diminished[19]. We constructed a recombinant adenovirus to transfer KDR promoter controlled double suicide gene into HUVEC cells. Prodrug sensitive experiments were performed to value the killing effects of the fusion double suicide gene under regulation of KDR promoters and mediated by an adenovirus vector on HUVEC cells.
Shutter plasmid pAdtrack, adenoviral backbone plasmid pAdEasy-1 and E. coli BJ5183 were provided by Dr. Belt Vogelstein of Johns Hopkins Oncology Center, Howard Hughes Institute of Medicine. pMD18-T vector was purchased from TaKaRa Biotechnology (Dalian) Co., Ltd. 293 cells and HUVEC cells were obtained from American Type Culture Collection (ATCC). All sorts of exonuclease enzyme, T4 DNA ligase, Taq DNA polymerase were purchased from New England Biolabs Co. DMEM, fetal bovine serum (FBS), transfection reagents, Lipofectamine 2000 were products of Gibco Co. Primers of KDR promoter, CD, TK genes were synthesized and sequenced by Sangon Biotechnology (Shanghai) Co., Ltd.
Primers for polymerase chain reaction (PCR) amplifying KDR promoter gene (including the minimus core of the gene sequences-226-+268) were designed according to the sequences provided by GenBank X89776. The upstream primer sequence 5’-GGAAGATCTAGTTGCTCAGC-GCCCGTTAC-3’, the downstream primer sequence 5’-CCCAAGCTTGGCGAAATGCCCAGAACTCG-3’, and BglII, HindIII cutting sites were added on 5’ end or 3’ end, respectively. Human blood genome was extracted and used as a template, the products were linked to a pMD18-T vector to construct pMD-18KDR.
CD and TK genes were also cloned (the upstream primer sequence of CD: 5’-AAGCTTAGGCTAGCAATGTC-GAATAACGCT-3’; the downstream primer sequence of CD: 5’-GGATCCTCCACGTTTGTAATCGATGGCTTC-3’; the upstream primer sequence of TK: 5’-GGATCCGG-CGGGGGCGGTGGAGGAGGGGGTATGGCTTCGTAC-3’; the downstream primer sequence of TK: 5’-TCTAGAT-TAGTTAGCCTCCCCCATCTC-3’) using the chromatosome DNA of E.coli JM109 and plasmid pREP8-TK as templates. Some transforms were made to make the initiation codon of the resulting CD gene to be ATG, termination code on TAG to be GGA which was to encode glycine, and its 5’ end, 3’ end to have BglIIand HindIII cutting sites, respectively. The two amplified segments were inserted into the pcDNA3 vector generating pcDNA3-CDglyTK.
pMD-18KDR, pcDNA3-CDglyTK were digested with BglII and HindIII. The resulting KDR, pcDNA3-CDglyTK fragments were purified and linked to each other to construct pcDNA3-KDR-CDglyTK, which was digested with BglII and PvuII after amplified in E. coli Top 10 bacteria to get KDR-CDglyTK fragments. After being digested with BglII and PvuII, pAdtrack was linked to KDR-CDglyTK fragments and pAdtrackKDR-CDglyTK was constructed.
pAdEasey-1 plasmid was transformed into E. coli BJ5183, then transformants were grown on LB agar plates containing ampicillin and strepolin. The transformed bacteria were named “AdEasey-1 bacteria”.
pAdtrackKDR-CDglyTK was linearized and transformed into AdEasey-1 bacteria, transformants were selected on LB agar plates containing 25 μg/mL kanamycin. Plasmid DNA was prepared from individual colonies, and agarose gel electrophoresis was performed. The correct recombinants could be clearly identified by size, as only the 11.2-kb pAdtrackKDR-CDglyTK plasmid and the 37-kb recombinant were selectable by kanamycin resistance.
The recombinant adenoviruses digested with PacI enzyme, were transferred into 293 cells mediated by a Lipofectamine2000 vector. Propagations of the recombinant viruses were visualized under a fluorescence microscope by GFP expression of transgenes, adenovirus supernate was ultracentrifuged in CsCl gradient to purify the viruses, and the titration of AdKDR-CDglyTK was measured with plaque formation assay.
The recombinant viruses were boiled and used as templates, two PCRs were performed, one was to make sure there were CDglyTK genes in the viruses, in which the upstream primer sequence of CD and the downstream primer sequence of TK were used, the other was to make sure if there were KDR promoter genes in them, primers of KDR promoter were used.
HUVEC cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and maintained in calorstat at 37 °C, 50 mL/L CO2. Cells (2×105/well, six-well plates, inoculated a day before) were infected with AdKDR-CDglyTK at a different multiplicity of infection (MOI). Percentages of cells expressing GFP were counted under a fluorescence microscope for 3 d.
HUVEC cells (1×104 cells/well, 96-well plates, inoculated a day before) were infected with AdKDR-CDglyTK at MOI of 100. Sixteen hours later, the medium was replaced with a fresh medium containing different concentrations of 5-FC and/or GCV, cells were cultured in the presence of prodrugs for 72 h. Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay according to the manufacturer’s protocol.
HUVEC cells (1×104 cells/well, 96-well plates, inoculated a day before) were infected with AdKDR-CDglyTK at different MOI. Sixteen hours later, the medium was replaced with a fresh medium containing GCV (100 µg/mL) and 5-FC (1000 µg/mL), cells were cultured in the presence of prodrugs for 72 h. Cell viability was determined by MTT assay.
Statistical analyses were made by ANOVA and LSD test. The values were calculated as mean±SD. P<0.05 was considered statistically significant.
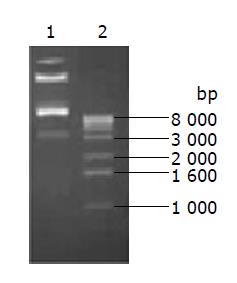
The products of PCR amplifying KDR promoter genes, CD and TK genes were sequenced and verified by Sangon Biotechnology (Shanghai) Co., Ltd. These segments were subcloned into pAdtrack. Figure 1 is a map of the resulting pAdtrackKDR-CDglyTK plasmid digested by BglII+Xba1.
The transformant of transforming AdtrackKDR-CDglyTK plasmid into Adeasey-1 bacteria was selected on LB agar plates containing ampicillin and strepolin, 17 clones were selected, 16 of which were proved to have been correctly recombined. The correct rate was 94.1% (16/17).
Three days after transferring pAdKDR-CDglyTK into 293 cells, we found that most transferred 293 cells expressed GFP. The titer of purified viruses after being sufficiently propagated was 4.2×1012 pfu/L.
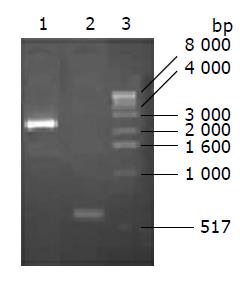
The products of PCR protocols using the recombinant viruses as a template were proved to be correct by size co-mparison (Figure 2), while KDR promoter was 580-bp and CDglyTK 2.5-bp.
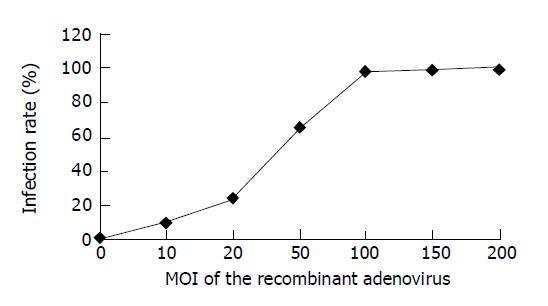
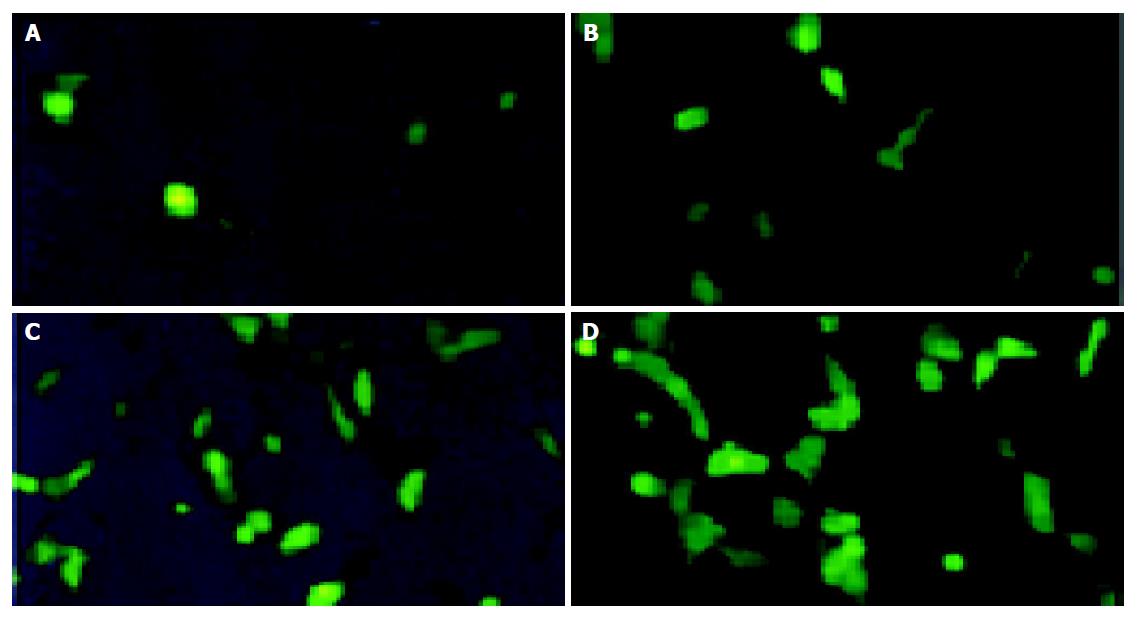
HUVEC cells infected with viruses at different MOI were observed in 3 d after infection (Figures 3 and 4). It indicated that the cell infection rate increased with the increase of MOI of the viruses: MOI = 1, only a few cells expressed GFP; MOI = 100, 97.8% cells expressed GFP; MOI = 200, almost all cells expressed GFP.
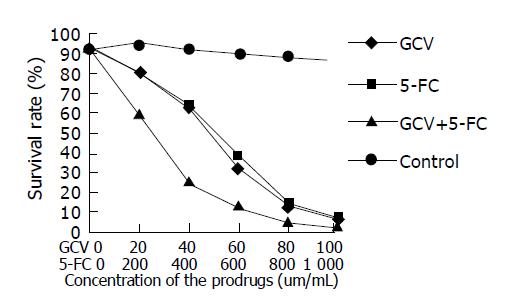
HUVEC cells infected with AdKDR-CDglyTK at MOI of 100 were maintained in culture medium containing different concentrations of GCV and/or 5-FC for 3 d, and survival rates were measured (Figure 5). The data indicated that transgene cells were highly sensitive to prodrugs, 93.7% transgene cells were killed when they were treated with 100 µg/mL GCV, 92.2% were killed when treated with 1000 µg/mL 5-FC, and 98.5% were killed when treated with GCV(100 µg/mL)+5-FC(1000 µg/mL). The cell survival rate decreased with the increase of concentration of prodrugs. The two prodrugs showed similar toxicities, however, a marked decrease in cell survival was observed when GCV and 5-FC were used in combination (P<0.05).
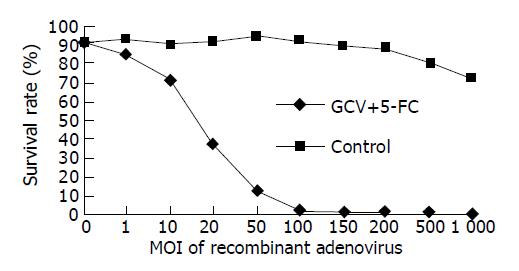
Multiplicity of infection of the virus also played an effective role in the killing. Combined GCV and 5-FC achieved a greater killing effect with increasing MOI, and the data of the control group (infected cells were maintained in culture medium without prodrugs) indicated that the virus itself had no high toxicity (Figure 6).
CD and TK are two competent suicide genes, but the results of numerous investigations aiming at eradicating tumors employing either CD or TK demonstrated limitations. Whether the limitations stemmed from unfavorable pharma-cokinetics, loss of transgene expression or biochemical resistance is not certain. On the other hand, the fusion genes of CD and TK presented an exciting superiority in many former studies[14-17]. CDglyTK encodes a bifunctional enzyme possessing both CD and TK specific activities, cytotoxicity could be enhanced by concurrently treating CDglyTK-expressing cells with 5-FC and GCV, resulting in a slight synergistic effect. More than that, studies indicated that limitations placed upon single suicide gene therapy by cell-specific prodrug sensitivities, such as TK which is far less effective than CD in killing pulmonary adenocarcinoma cells[20], might be overcome by use of bifunctional CDglyTK enzyme because it could act through whatever prodrug-mediated cytotoxic pathway existing in a given cell type[21]. As it has been established that the “bystander effect” of a suicide gene is cell-type-specific[20,22], it also may be overcome by use of CDglyTK fusion genes of the same mechanism, and if both CD and TK mediated bystander killing pathways are available within the same tumor cell type, both can be utilized by the fusion genes. A further benefit of the fusion genes is its ability to circumvent the acquired drug resistance[14,15]. Acquired prodrug resistance could be easily overcome through the use of CDglyTK gene because it is highly improbable that tumor cells would develop biochemical resistance to both 5-FC and GCV, except via loss of fusion gene expression.
Vascular endothelial growth factor (VEGF) is a potent and specific mitogen for endothelial cells. It plays a major role in angiogenesis and vasculogenesis. KDR and Flt-1 are the two receptor tyrosine kinases that regulate the actions of VEGF and are expressed in endothelial cells, while the related receptor Flt-4, has been found on lymphatic endothelium. The expression pattern of Flt-4 suggested it might play a role during lymphangiogenesis[23,24]. KDR was critically involved in the regulation of angiogenesis, both in developing and adult animals[25]. Vascular endothelial cells were renewed at a low speed in normal conditions, and its KDR expression level was very low, while tumor vascular endothelial cells proliferated quickly and its KDR expression level was 500× higher than that of vascular endothelial cells of normal tissues[26]. Herein, the targeted expression of therapeutic genes in tumor vascular endothelial cells by transcriptional regulation of KDR promoter, can markedly reduce the toxicity and side effects of gene therapy targeting vascular endothelial cells of tumors.
Adenovirus vector system has many advantages. Adenovirus vectors can be prepared at much higher titres than retroviral vectors and have a high efficiency of gene transfer regardless of the proliferative state of tissues whereas retroviral vectors insert their genes only into dividing cells. Adenovirus genomes usually do not integrate into the host cell chromosomes. Although the duration of in vivo gene expression with an adenovirus vector is short, the level of therapeutic gene expression is much higher, which is of much importance in suicide gene therapy because the therapeutic effect of suicide genes is to kill the transgene cells and cells nearby, a transient and high level expression would be enough, as once its killing effect realizes that the gene would be inactivated due to the death of the host cells, and a long expression duration would be meaningless.
In our experiment, recombinant adenoviruses infected HUVEC cells effectively, 97.8% cells were infected and expressed GFP when infected with viruses at MOI = 100. Almost all cells expressed GFP when MOI = 200. Transgene cells were highly sensitive to both 5-FC and GCV, and both prodrugs showed a similar toxicity. However, a marked decrease in cell survival was observed when GCV and 5-FC were used in combination when we treated the transgene cells with all concentrations of prodrugs. This phenomenon existed when we treated the transgene cells. The data also indicate that cell survival rate decreased with increasing either the concentration of prodrugs or MOI of the virus. In addition, the results of survival rate detected 3 d after HUVEC cells were infected with recombinant adenoviruses at different MOI showed no significant difference, indicating that the recombinant virus itself is not much toxic to HUVEC cells.
| 1. | Jakóbisiak M, Lasek W, Gołab J. Natural mechanisms protecting against cancer. Immunol Lett. 2003;90:103-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3254] [Cited by in RCA: 3194] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 3. | Pastorino F, Brignole C, Marimpietri D, Cilli M, Gambini C, Ribatti D, Longhi R, Allen TM, Corti A, Ponzoni M. Vascular damage and anti-angiogenic effects of tumor vessel-targeted liposomal chemotherapy. Cancer Res. 2003;63:7400-7409. [PubMed] |
| 4. | Mentlein R, Held-Feindt J. Angiogenesis factors in gliomas: a new key to tumour therapy? Naturwissenschaften. 2003;90:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Li Y, Jin Y, Chen H, Jie G, Tobelem G, Caen JP, Han ZC. Suppression of tumor growth by viral vector-mediated gene transfer of N-terminal truncated platelet factor 4. Cancer Biother Radiopharm. 2003;18:829-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Lamszus K, Kunkel P, Westphal M. Invasion as limitation to anti-angiogenic glioma therapy. Acta Neurochir Suppl. 2003;88:169-177. [PubMed] |
| 7. | Yang SH, Lin JK, Chen WS, Chiu JH. Anti-angiogenic effect of silymarin on colon cancer LoVo cell line. J Surg Res. 2003;113:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Longo R, Sarmiento R, Fanelli M, Capaccetti B, Gattuso D, Gasparini G. Anti-angiogenic therapy: rationale, challenges and clinical studies. Angiogenesis. 2002;5:237-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Fan YF, Huang ZH. Progress in the studies of gene therapy for colorectal cancer. Shijie Huaren Xiaohua Zazhi. 2001;9:427-430. |
| 10. | Jiao LR, Havlik R, Nicholls J, Jensen SL, Habib NA. Suicide gene therapy in liver tumors. Methods Mol Med. 2004;90:433-450. [PubMed] |
| 11. | Miyagi T, Koshida K, Hori O, Konaka H, Katoh H, Kitagawa Y, Mizokami A, Egawa M, Ogawa S, Hamada H. Gene therapy for prostate cancer using the cytosine deaminase/uracil phosphoribosyltransferase suicide system. J Gene Med. 2003;5:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Brown NL, Lemoine NR. Clinical trials with GDEPT: cytosine deaminase and 5-fluorocytosine. Methods Mol Med. 2004;90:451-457. [PubMed] |
| 13. | Porosnicu M, Mian A, Barber GN. The oncolytic effect of recombinant vesicular stomatitis virus is enhanced by expression of the fusion cytosine deaminase/uracil phosphoribosyltransferase suicide gene. Cancer Res. 2003;63:8366-8376. [PubMed] |
| 14. | Rogulski KR, Kim JH, Kim SH, Freytag SO. Glioma cells transduced with an Escherichia coli CD/HSV-1 TK fusion gene exhibit enhanced metabolic suicide and radiosensitivity. Hum Gene Ther. 1997;8:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Jiang YR, Ma DX, Chen XL, Liu CS. Killing effect of double suicide genes mediated by lentivirus on lymphoma cells. AiZheng. 2003;22:916-921. [PubMed] |
| 16. | Li XJ, Wang KM, Xu Y, Wang ZH, Xia AD, Chen SS, Qian GX. Ionizing radiation-regulated killing of human hepatoma cells by liposome-mediated CDglyTK gene delivery. ShengWu HuaXue Yu ShengWu WuLi XueBao (Shanghai). 2003;35:64-70. [PubMed] |
| 17. | Corban-Wilhelm H, Becker G, Bauder-Wüst U, Greulich D, Debus J. Cytosine deaminase versus thymidine kinase: a comparison of the antitumor activity. Clin Exp Med. 2003;3:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Ear T, Giguère P, Fleury A, Stankova J, Payet MD, Dupuis G. High efficiency transient transfection of genes in human umbilical vein endothelial cells by electroporation. J Immunol Methods. 2001;257:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Patterson C, Perrella MA, Hsieh CM, Yoshizumi M, Lee ME, Haber E. Cloning and functional analysis of the promoter for KDR/flk-1, a receptor for vascular endothelial growth factor. J Biol Chem. 1995;270:23111-23118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Hoganson DK, Batra RK, Olsen JC, Boucher RC. Comparison of the effects of three different toxin genes and their levels of expression on cell growth and bystander effect in lung adenocarcinoma. Cancer Res. 1996;56:1315-1323. [PubMed] |
| 21. | Rogulski KR, Kim JH, Kim SH, Freytag SO. Glioma cells transduced with an Escherichia coli CD/HSV-1 TK fusion gene exhibit enhanced metabolic suicide and radiosensitivity. Hum Gene Ther. 1997;8:73-85. |
| 22. | Trinh QT, Austin EA, Murray DM, Knick VC, Huber BE. Enzyme/prodrug gene therapy: comparison of cytosine deaminase/5-fluorocytosine versus thymidine kinase/ganciclovir enzyme/prodrug systems in a human colorectal carcinoma cell line. Cancer Res. 1995;55:4808-4812. [PubMed] |
| 23. | Thakker GD, Hajjar DP, Muller WA, Rosengart TK. The role of phosphatidylinositol 3-kinase in vascular endothelial growth factor signaling. J Biol Chem. 1999;274:10002-10007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 177] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA. 1995;92:3566-3570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1026] [Cited by in RCA: 1044] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 25. | Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997;272:23659-23667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 538] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 26. | Folkman J. The role of angiogenesis in tumor growth. Semin Cancer Biol. 1992;3:65-71. [PubMed] |