Published online Jun 28, 2005. doi: 10.3748/wjg.v11.i24.3675
Revised: June 29, 2004
Accepted: August 5, 2004
Published online: June 28, 2005
AIM: To establish a simplified and reliable animal model of insulin resistance with low cost in Wistar rats.
METHODS: Wistar rats were treated with a high fat emulsion by ig for 10 d. Changes of the diets, drinking and body weight were monitored every day and insulin resistance was evaluated by hyperinsulinemic-euglycemic clamp techniques and short insulin tolerance test using capillary blood glucose. Morphologic changes of liver, fat, skeletal muscles, and pancreatic islets were assessed under light microscope. mRNA expressions of GLUT2 and α-glucosidase in small intestine epithelium, GLUT4 in skeletal muscles and Kir6.2 in beta cell of islets were determined by in situ hybridization.
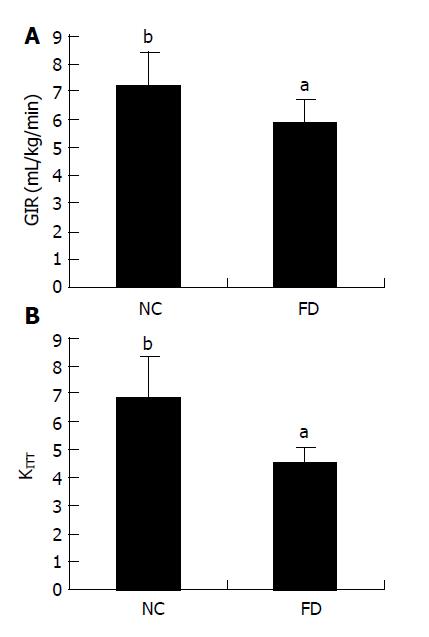
RESULTS: KITT was smaller in treated animals (4.5±0.9) than in untreated control Wistar rats (6.8±1.5), and so was glucose injection rate. Both adipocyte hypertrophy and large pancreatic islets were seen in high fat fed rats, but no changes of skeletal muscles and livers were observed. mRNA levels of GLUT2, α-glucosidase in small intestinal epithelium and Kir6.2 mRNA in beta cells of islets increased, whereas that of GLUT4 in skeletal muscles decreased in high fat fed group compared with normal control group.
CONCLUSION: An insulin resistance animal model in Wistar rats is established by ig special fat emulsion.
- Citation: Ai J, Wang N, Yang M, Du ZM, Zhang YC, Yang BF. Development of Wistar rat model of insulin resistance. World J Gastroenterol 2005; 11(24): 3675-3679
- URL: https://www.wjgnet.com/1007-9327/full/v11/i24/3675.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i24.3675
Epidemiological studies suggest that insulin resistance is not only an independent risk factor that induces type 2 diabetic mellitus, but also the common cause of hypertension, coronary heart disease, and cerebral vessel disease, thus the key to cure and prevent heart and cerebral vessel disease. It is of paramount importance to establish an insulin resistance animal model, in order to have a better understanding of the pathological process of insulin resistance and to develop therapeutic drugs. Several insulin resistance animal models are available, including hereditary ob/ob mice and SD or Wistar rat models developed by either injecting low-dose dexamethasone (2 μg/d) into abdominal cavity or feeding food rich in fructose and sucrose[1-7]. The major disadvantages of all these animal models are the long experimental cycles (4-30 wk) and the less relevant pathologic status of insulin resistance induced by a sole factor as opposed to the natural multi-factoral process. The present study was to establish an insulin resistance animal model using Wistar rats with more clinically relevant pathophysiological characteristics of insulin resistance based on glucose utility of the body and alterations of various cellular and molecular events related to insulin resistance.
Male Wistar rats weighing 180-220 g were obtained from Department of Animals, Harbin Medical University. Insulin was purchased from the First Biochemical Drug Company of Shanghai. The kit for in situ hybridization of small-intestine GLUT2, α-glucosidase, Kir6.2 in islet beta cells and GLUT4 in skeletal muscles was purchased from BOSD Biotech, Wuhan, China.
A constant volume of 100 mL fat emulsion containing 20 g lard, 1 g thyreostat, 5 g cholesterol, 1 g sodium glutamate, 5 g sucrose and 5 g saccharose, 20 mL Tween 80, 30 mL propylene glycol was prepared by adding distilled water and stored at 4 °C.
Twenty-four Wistar rats were randomly divided into normal control group and high fat emulsion group, 12/group. Rats in normal control group received common water, rats in high fat emulsion group received fat emulsion for 10 d.
Rats were weighed and placed into mouse cage after fasting overnight. Blood sugar in rats was detected six times after ip insulin (0.05 U/kg) using a blood sugar detector. Abscissa indicates time and ordinate expresses nature logarithm of blood sugar. Regression coefficient (r) or slope was determined by linear regression and KITT was calculated by multiplying r by 100. K value indicates insulin sensibility with smaller K values for lower sensibilities.
As described previously[9], food was withdrawn 12 h before the experiment. The rats were then anesthetized by ip amobarbital sodium (25 mg/kg) after they had been weighed. Rats were cannulated in the jugular vein for infusion of glucose and insulin (dual cannula) and in the carotid artery for sampling. All cannulae were tunneled subcutaneously, and encased in silastic tubing (0.08 cm) sutured to the skin. After infusion of glucose (10%) and insulin (1 IU/mL) from dual cannula (constant velocity), blood sugar was measured. To keep the blood sugar in a relatively steady state, the rate of glucose infusion was continuously adjusted. Glucose injection rate (GIR) was measured under homeostasis six times during the experiment.
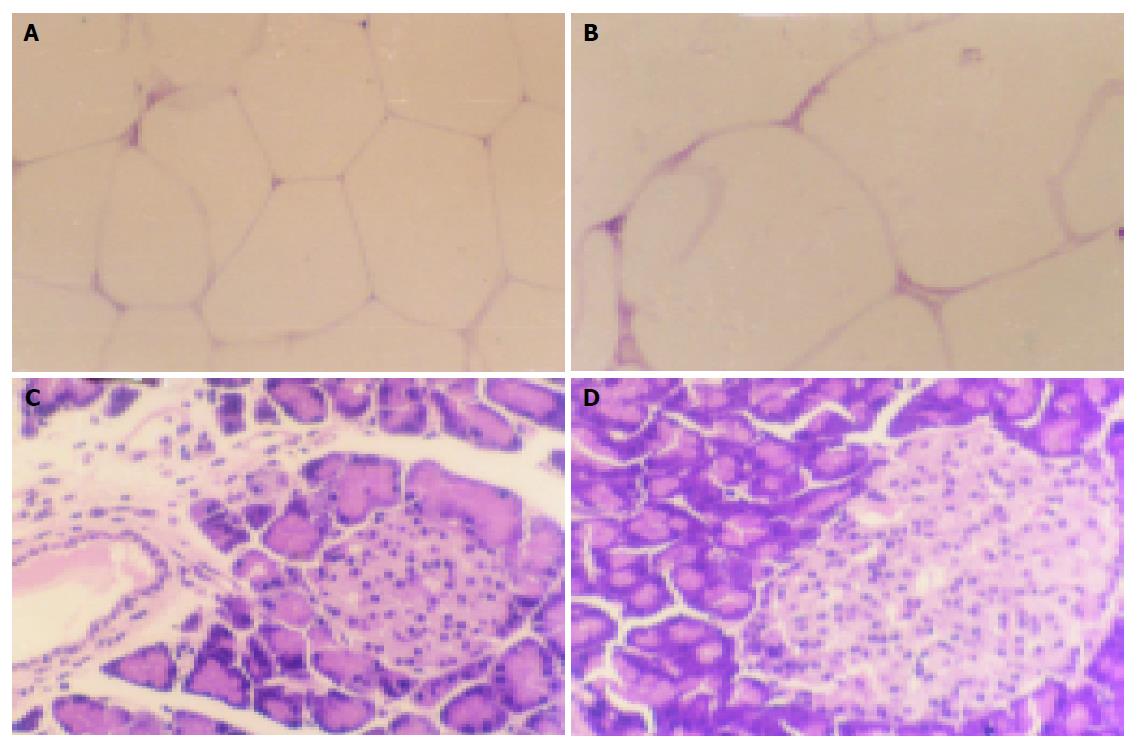
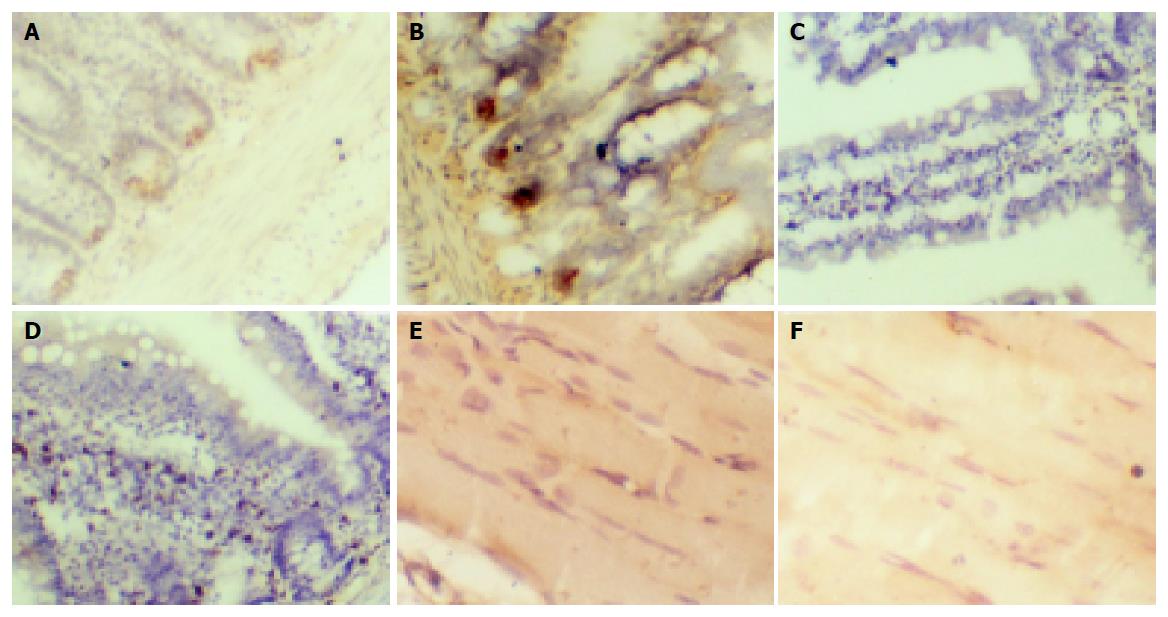
After rats were fasted for 3 h, perirenal fat, gastrocnemius muscle, liver, and pancreas were dissected. The tissues were then fixed with 4% paraformaldehyde, embedded in paraffin wax, sectioned, and stained with eosin by sappan wood. Morphological alterations were examined under a light microscope. The procedure for mRNA levels was described previously. The positive expression of α-glucosidase and Kir6.2 showed brown staining signals in villi of small intestine and in pancreatic islet β-cells. GLUT2 mRNA was expressed in the incisurae between two villi of small intestine and in cytoplasm of skeleton muscle. Expression level was assayed by the degree of color.
Data were analyzed by Student’s t-test. The results were expressed as mean±SD.
Compared with normal control group, the drinking, body weight, and visceral fat increased markedly 10 d after ig fat emulsion (Table 1, P<0.05). In contrast, no changes were observed in diet group.
The results of short insulin tolerance test using capillary blood glucose revealed that KITT decreased to 4.5±0.9 in rats treated with fat emulsion (ig, 10 d) (P<0.05, n = 12). Our hyperinsulinemic-euglycemic clamp test indicated that the GIR for keeping homeostasis of blood sugar in rats of high fat emulsion group was decreased in fat treated group (Figure 1A).
No morphological changes were observed in skeletal muscle and liver, but in larger adipocytes and pancreas islets when the tissues were stained with HE in high fat emulsion group compared to control group (Figure 2).
Both GLUT2 and α-glucosidase mRNAs in small intestinal epithelium were increased in fat emulsion group compared to control group (Figures 3A-3D). The number of cells expressing α-glucosidase mRNA in high fat emulsion group was 50±6 and 40±5 in control group (P<0.05, n = 12). GLUT2 mRNA was diffused in the incisurae between two villi of small intestine. In the sight of 100 cm2, the expression area of GLUT2 mRNA in high fat emulsion group was increased to 3.8±0.6 cm2 from 3.0±0.4 cm2 in control group (P<0.05, n = 12). In cytoplasm of skeleton muscle of fat emulsion group, the expression of GLUT4 mRNA was lower, as indicated by the brighter brown color, than in control group (Figures 3E and 3F). Kir6.2 mRNA in β-cells showed a tendency to increase in fat emulsion group because its positive expression density did not change but the volume increased compared to control group.
In the present study, we developed a new formula of fat emulsion based on the insulin-resistant animal models established by other laboratories. The formula for preparing fat emulsion is to combine high fat diets with fructose and sucrose. Moreover, the fat emulsion was administered to rats by ig but not by food feeding so as to control the daily fat intake. The possible imbalance of fat intake due to decreased appetite as a result of high fat diets was avoided. Increased drinking accompanying an increase in body weight and visceral fat was observed though the rats were administered high fat emulsion for 10 d. This is typical of the clinical phenotype.
In order to clarify if the insulin-resistant animal model was established in our study, we detected the dynamic characteristics of blood sugar after insulin injection by short insulin tolerance test using capillary blood glucose. The result demonstrated that the KITT value decreased markedly after ig fat emulsion for 10 d compared to control group, indicating that the rats are insensitive to exogenous insulin, i.e., insulin resistance.
Insulin resistance refers to the insensitivity of tissues (such as skeletal muscle, liver, kidney, and adipose tissue) to insulin action, i.e., the weaker glucose utilization of body after insulin action that results in hyperglycemia. The classical test used to evaluate insulin resistance is hyperinsulinemic-euglycemic clamp experiment[10,11]. We determined the insulin resistance using hyperinsulinemic-euglycemic clamp technique that is known to be the most reliable method for verifying whether or not insulin resistance is achieved in rats after feeding fat emulsion. According to negative feedback mechanism, if we want to keep blood sugar in the basal level by changing injection rate of exogenous glucose, we should regulate the injection rate of exogenous insulin. The exogenous GIR is equal to the glucose utilization rate of peripheral organs because the endogenous glucose production (from liver) could be completely inhibited when the plasma insulin level is excessively high. Hence, GIR can be considered as an index for evaluating the action of insulin on peripheral organs.
Our data showed that the GIR was indeed decreased in rats treated with high fat emulsion, indicating that insulin resistance is achieved in fat emulsion group.
Diabetes mellitus is a pathological process affecting the whole body system. Skeletal muscle, fat, and liver are considered as the insulin-sensitive tissues[12-16]. Alterations of the functional status of these tissues may result in insulin resistance of the body. The main manifestation is the dysfunction of glucose absorption and utilization, metabolism disturbance of glucose in liver cells and lipo-metabolism disturbance in adipose cells[15,17]. Several papers have reported increases in adipose cell volume and number accompanied with redistribution of these cells over the body in insulin-resistant animals and humans. Nevertheless, it is more important that adipose cell volume increases when insulin resistance occurs[14]. Our experiments demonstrated that insulin resistance could be induced in rats after being treated with high fat emulsion by ig. However, we still do not know if morphological changes occur as a result of dysfunction. We therefore carried out histopathological assay by HE staining of insulin-sensitive tissues, such as fat, skeletal muscle, and liver. We found no morphological changes in both skeletal muscle and liver, but adipose cell volume was markedly enlarged in rats of fat emulsion group. In high fat emulsion group, pancreatic islets enlarged but no changes in the other part of pancreas were seen. The morphological changes in both fat cells and pancreatic islets in high fat emulsion group confirmed the success of our insulin-resistant animal model.
Glucose is an important substance that keeps the balance of energy metabolism and life. Glucose is a polarity molecule that cannot pass the lipid bilayer of cell membrane by free diffusion. Small intestine and renal tubule can absorb glucose and other tissues must intake glucose by facilitating diffusion with glucose transporter proteins in cell membrane. Among the various glucose transporter proteins, GLUT2 and GLUT4 have been more intensively studied.
GLUT2 is an important glucose transporter protein which distributes in hepatocytes, pancreatic islet β-cells, small intestine, and kidney[18-20]. People pay close attention to GLUT2 because it is related to pathogenesis of diabetes mellitus. GLUT2 expression in pancreatic islet β-cells decreases in BB rats and Zuker obesity rats with autoimmune diabetes mellitus. Some studies reported that GLUT2 mRNA and protein expressions are enhanced in the liver of diabetes mellitus rats induced by streptozotocin, but others found no changes. Studies on expression of GLUT2 in epithelial cells found that GLUT2 protein expression in sarcolemma of small intestine increases in both STZ-induced diabetes mellitus rats and high sugar feeding rats, but no changes occurred in hyperglycemia rats caused by glucose filling, while glucose transporter activity increases in these conditions[21-23]. Until now, study on mRNA expression of GLUT2 in small intestine of insulin-resistant rats has not been reported.
GLUT4 is an insulin reaction protein transporter which mainly distributes in adipose cells and striated muscles[24]. On the one hand, GLUT4 is the richest protein transporter in both adipose cells and striated muscles. On the other hand, GLUT4 expression level is coincident with glucose utilization ability of various types of adipose cells. Almost all the glucose absorbed by muscles gets converted into glycogen in high insulin condition. Glucose transport of muscular tissues is very important for the glucose utility of body because glucose transport is the rate-limiting step of glucose utility of muscle tissue. To date, studies have evidenced that not only the protein expression of GLUT4 decreases in insulin-resistant condition, but also the translocation of GLUT4 from cytoplasm to cell membrane is defected[25].
Based on the above reasons, we measured mRNA expression of GLUT2 in small intestinal epithelial cells and GLUT4 in gastrocnemius muscle in our insulin-resistant rat model. On the one hand, we detected mRNA expression of GLUT2 in small intestinal epithelial cells. On the other hand, we further verified at molecule level that we successfully established the insulin-resistant animal model. Our study demonstrated that mRNA expression of GLUT2 in small intestinal epithelial cells increased in high fat emulsion group compared with normal control group. In contrast, GLUT4 mRNA expression decreased. The results reveal that the insulin-resistant animal model is successfully established.
In order to ensure that sugar can be absorbed in small intestine, polysaccharide must be divided into monosaccharide by α-glucose glycosidase of small intestine. In our experiments, mRNA expression of α-glucose glycosidase in small intestine increased in fat emulsion group. The mRNA expression of β-cell Kir6.2 in pancreatic islets did not change in fat emulsion group, but the total expression level increased because of the enlargement of pancreatic islet volume.
In conclusion, an insulin-resistant rat model is established by ig special fat emulsion.
Co-correspondents: Jing Ai
Science Editor Wang XL Language Editor Elsevier HK
| 1. | Severino C, Brizzi P, Solinas A, Secchi G, Maioli M, Tonolo G. Low-dose dexamethasone in the rat: a model to study insulin resistance. Am J Physiol Endocrinol Metab. 2002;283:E367-E373. [PubMed] |
| 2. | Pénicaud L, Berthault MF, Morin J, Dubar M, Ktorza A, Ferre P. Rilmenidine normalizes fructose-induced insulin resistance and hypertension in rats. J Hypertens Suppl. 1998;16:S45-S49. [PubMed] |
| 3. | Lombardo YB, Drago S, Chicco A, Fainstein-Day P, Gutman R, Gagliardino JJ, Gomez Dumm CL. Long-term administration of a sucrose-rich diet to normal rats: relationship between metabolic and hormonal profiles and morphological changes in the endocrine pancreas. Metabolism. 1996;45:1527-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Baptista T, Alvarez L, Lacruz A, de Mendoza S, Hernández L. Glucose tolerance and serum insulin levels in an animal model of obesity induced by sub-acute or chronic administration of antipsychotic drugs. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Haluzik M, Colombo C, Gavrilova O, Chua S, Wolf N, Chen M, Stannard B, Dietz KR, Le Roith D, Reitman ML. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology. 2004;145:3258-3264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Elton CW, Pennington JS, Lynch SA, Carver FM, Pennington SN. Insulin resistance in adult rat offspring associated with maternal dietary fat and alcohol consumption. J Endocrinol. 2002;173:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Mangaloglu L, Cheung RC, Van Iderstine SC, Taghibiglou C, Pontrelli L, Adeli K. Treatment with atorvastatin ameliorates hepatic very-low-density lipoprotein overproduction in an animal model of insulin resistance, the fructose-fed Syrian golden hamster: evidence that reduced hypertriglyceridemia is accompanied by improved hepatic insulin sensitivity. Metabolism. 2002;51:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Zhang JQ, Gao CR, Huang QL. Deter mination of Insulin Sensitivity by Short Insulin Tolerance Test Using Capillary Blood Glucose. Chin J Endocrinol Metab. 1997;13:77-80. |
| 9. | Xu SY, Bian RL. Experiment Mothed of Pharmacology, Third Edition. Beijing: People's Medical Publishing House 2002; 1522-1523. |
| 10. | Straczkowski M, Dzienis-Straczkowska S, Szelachowska M, Kowalska I, Stepień A, Kinalska I. Insulin resistance in obese subjects with impaired glucose tolerance. Studies with hyperinsulinemic euglycemic clamp technique. Pol Arch Med Wewn. 2003;109:359-364. [PubMed] |
| 11. | Saruç M, Yüceyar H, Ayhan S, Türkel N, Tuzcuoglu I, Can M. The association of dehydroepiandrosterone, obesity, waist-hip ratio and insulin resistance with fatty liver in postmenopausal women--a hyperinsulinemic euglycemic insulin clamp study. Hepatogastroenterology. 2003;50:771-774. [PubMed] |
| 12. | Klaus S. Adipose tissue as a regulator of energy balance. Curr Drug Targets. 2004;5:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Faraj M, Lu HL, Cianflone K. Diabetes, lipids, and adipocyte secretagogues. Biochem Cell Biol. 2004;82:170-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Das M, Gabriely I, Barzilai N. Caloric restriction, body fat and ageing in experimental models. Obes Rev. 2004;5:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Flück CE, Slotboom J, Nuoffer JM, Kreis R, Boesch C, Mullis PE. Normal hepatic glycogen storage after fasting and feeding in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2003;4:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 893] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 17. | Cohen SE, Tseng YH, Michael MD, Kahn CR. Effects of insulin-sensitising agents in mice with hepatic insulin resistance. Diabetologia. 2004;47:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Chen X, Patil JG, Lok SH, Kon OL. Human liver-derived cells stably modified for regulated proinsulin secretion function as bioimplants in vivo. J Gene Med. 2002;4:447-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Cui XL, Jiang L, Ferraris RP. Regulation of rat intestinal GLUT2 mRNA abundance by luminal and systemic factors. Biochim Biophys Acta. 2003;1612:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Hosokawa M, Dolci W, Thorens B. Differential sensitivity of GLUT1- and GLUT2-expressing beta cells to streptozotocin. Biochem Biophys Res Commun. 2001;289:1114-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Santer R, Hillebrand G, Steinmann B, Schaub J. Intestinal glucose transport: evidence for a membrane traffic-based pathway in humans. Gastroenterology. 2003;124:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Marks J, Carvou NJ, Debnam ES, Srai SK, Unwin RJ. Diabetes increases facilitative glucose uptake and GLUT2 expression at the rat proximal tubule brush border membrane. J Physiol. 2003;553:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Au A, Gupta A, Schembri P, Cheeseman CI. Rapid insertion of GLUT2 into the rat jejunal brush-border membrane promoted by glucagon-like peptide 2. Biochem J. 2002;367:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Watson RT, Pessin JE. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog Horm Res. 2001;56:175-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 161] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Brennan CL, Hoenig M, Ferguson DC. GLUT4 but not GLUT1 expression decreases early in the development of feline obesity. Domest Anim Endocrinol. 2004;26:291-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |