Published online Jun 28, 2005. doi: 10.3748/wjg.v11.i24.3660
Revised: November 9, 2004
Accepted: December 3, 2004
Published online: June 28, 2005
AIM: To prepare a kind of magnetic iron-dextran nanoparticles that was coated with anti-E.coli O157:H7 IgG, analyze its application conditions, and try to use it to isolate E.coli O157:H7 from foods.
METHODS: Magnetic iron-dextran nanoparticles were prepared by the reaction of a mixture of ferric and ferrous ions with dextran polymers under alkaline conditions. The particles were coated with antiserum against E.coli O157:H7 by the periodate oxidation-borohydride reduction procedure. The oxidation time, amount of antibody coating the particles, amount of nanoparticles, incubation time and isolation time were varied to determine their effects on recovery of the organisms. Finally, the optimum conditions for isolating E.coli O157:H7 from food samples were established.
RESULTS: E.coli O157:H7 can be isolated from samples within 15 min with the sensitivity of 101 CFU/mL or even less. In the presence of 108 CFU/mL of other organisms, the sensitivity is 101-102 CFU/mL. Nonspecific binding of other bacteria to the particles was not observed. Two and a half hours of enrichment is enough for the particles to detect the target from the food samples inoculated with 1 CFU/g.
CONCLUSION: Isolation of target bacteria by immuno-magnetic nanoparticles is an efficient method with high sensitivity and specificity. The technique is so simple that it can be operated in lab and field even by untrained personnel.
-
Citation: Duan HL, Shen ZQ, Wang XW, Chao FH, Li JW. Preparation of immunomagnetic iron-dextran nanoparticles and application in rapid isolation of
E.coli O157:H7 from foods. World J Gastroenterol 2005; 11(24): 3660-3664 - URL: https://www.wjgnet.com/1007-9327/full/v11/i24/3660.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i24.3660
The enterohemorrhagic E.coli (EHEC) O157:H7 is recognized as an important human pathogen. The outbreaks of EHEC infections have been attributed to a variety of sources, mainly raw meat and drinking water. Illnesses caused by E.coli O157:H7 can range from self-limited watery diarrhea to life-threatening manifestations such as hemolytic uremic syndrome or thrombotic thrombocytopenic purpura. The lack of efficient selective enrichment medium for this particular strain of E.coli reduces the sensitivity and specificity of the conventional cultural method. However, the technique immunomagnetic separation (IMS) presents a physical selective enrichment procedure. When magnetic particles are covalently coupled with antibody specific to E.coli O157:H7, it is possible to easily achieve the aim of rapid isolation of the target organisms. The objective of the research was to prepare a kind of immunomagnetic nanoparticles, analyze its application conditions and try to use it to isolate E.coli O157:H7 from foods.
The polymer sample used in this study was commercial dextran with a molecular weight of 40000 (T-40) obtained from Pharmacia, Uppsala, Sweden. Sephacryl S-300 gel was available from Sigma Corporation. Ferric chloride hexahydrate, ferrous chloride tetrahydrate and other chemicals were from local suppliers and were of analytical grade. All the aqueous solutions were prepared by distilled water and filtered through 0.22-µm membrane.
Anti-O157:H7-specific antibody was prepared and purified in our laboratory.
Fifty-nine bacterial strains were used in this experiment. E.coli O157:H7 (strain 882364) was obtained from Institute of Epidemiology and Microbiology, Academy of Preventive Medical Sciences of China. Nineteen strains of other serotypes of E.coli, 6 strains of S.aureus, 16 strains of Salmonella, 14 strains of Shigella and 3 strains of Y.enterocolitica were all supplied by China Center for Type Culture Collection (CCTCC).
Ferromagnetic iron-dextran particles were prepared by the reaction of a mixture of ferric and ferrous ions with dextran polymers under alkaline conditions[1-4]. Routinely, under N2 purging, 20 mL of 500 g/L dextran T-40 in distilled water was mixed with the iron solution containing 16 mL of 0.7 mol/L FeCl3•6H2O and 4 mL of 1.6 mol/L FeCl2•4H2O, and kept at 60 °C for 15 min. With vigorous stirring, 40 mL of 5 mol/L ammonia solution was then added dropwise to the iron-polymer mixture, keeping at 60 °C for a further 15 min, with N2 protecting from oxidation. The resulting suspension was neutralized with acetic acid. The aggregates in the outcome were removed by centrifugation at 600 r/min for 15 min. Unbound dextran was separated from iron-dextran nanoparticles by gel filtration chromatography on a Sephacryl S-300 column, which was equilibrated with 0.01 mol/L phosphate buffer at pH 7.4[5-7]. Particles collected had a concentration of 8 mg/mL as determined by dry weight analysis.
In order to couple the particles with antibody, oxidation of dextran must be under a mild condition, generally using NaIO4 as the oxidant with a final concentration of about 5 mmol/L[8]. In this experiment, 0.25 mL of 25 mmol/L NaIO4 was used to oxidize 1 mL of Fe3O4-dextran nanoparticles. The reaction was kept away from light and oxygen, with a regular rotation of 150 r/min for a period of time. After that, 0.2 mL of 2 mol/L ethylene glycol was added and rotated for another half an hour to terminate oxidation. The excess periodate was removed by dialyzing the suspension for 24 h against 0.01 mol/L phosphate-buffered saline (PBS) at 4 °C.
After oxidation, part of hydroxyl groups had become aldehyde group, which displayed strong interactions with the amino groups of various compounds to form Schiff’s bases[9-11]. A different amount of antibody was added to the particle suspension, mixed thoroughly, and placed in dark at 4 °C for 8 h. Reduction with 0.5 mol/L NaBH4 for 30 min would contribute to form stable configuration. Uncoupled antibody was separated from the conjugates also by gel filtration chromatography on Sephacryl S-300 column as above.
E.coli O157:H7 suspension that was enriched in broth for 18 h followed by being diluted to 102 CFU/mL was chosen as the sample. Certain amount of magnetic nanoparticles were dispensed into 1.5 mL eppendorf tubes, and then 1 mL of the samples was added[12,13]. The tubes were closed and inverted several times. The samples were incubated for some time at room temperature, and rotation of about 20 r/min might bring the samples and the particles into closer contact. The particles were separated by placing a magnetic plate to the side wall of the tubes and the sample was kept for some time to concentrate the particles into a pellet on the side of the tubes. The tubes were opened carefully, aspirated and the sample supernatant as well as the remaining liquid in the cap was discarded. The magnetic plate was removed and 1 mL washing buffer (0.01 mol/L PBS pH 7.4 with the addition of 0.05% Tween-20) was added to remove nonspecific attachment. The particles were then separated again. As there existed the possibility that several bacteria could attach to one particle simultaneously and appear as one colony on the plate, the particle-bacteria complex in 50 µL of papain-PBS buffer (concentration of papain was 1.08 mg/mL) was resuspended and placed at 4 °C for 24 h, which helped antigen detach from antibody[14]. The suspension containing bacteria was transferred onto plates restrictive for E.coli O157:H7 (SMAC) to determine CFU (colony forming unit) by plate counting after an 18-h incubation at 37 °C. At the same time, the initial number of the bacteria in 1 mL samples was counted by standard procedure. Recovery could be obtained from their ratio. For further confirmation of the isolated micro-organism, biochemical or molecular biological method can be used regularly[15-20].
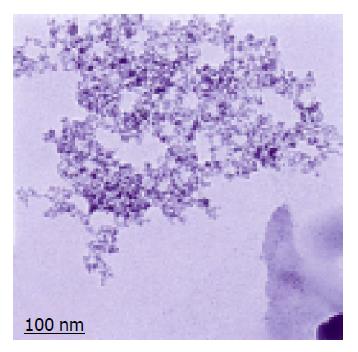
Magnetic iron-dextran nanoparticles were black suspension. They were roughly spherical in shape with a diameter ranging from 40 to 60 nm when observed under laser scattering system, and contained an electron dense core of 5 nm which enabled them to be seen by transmission electron microscopy (Figure 1). After purification, particle suspension with a concentration of 8 mg/mL was obtained. They were stable in physiological buffers and suspension over a pH range of 4-10 was maintained. The particles could be aggregated in the presence of a magnetic plate, and had no magnetic moment in the absence of magnetostatic field, that is, they possessed superparamagnetic properties.
Oxidation time is one of the important factors to determine the efficiency of linkage of antibody. In this paper, the nanoparticles were oxidized for 0.5-12 h (i.e., 0.5, 1, 2, 4, 6, 8, 10, 12 h), then connected with enough antibody (surplus antibody was removed by filtration). Now the particles could be used for detection (added enough particles into the sample, incubated for 30 min and magnetically separated for 5 min). Proper oxidation time could be determined by comparison of the recovery (Table 1).
The results indicated that relatively high recovery could be obtained when the particles were oxidized for 4-12 h, with the concentration of particles of 8 mg/mL, and oxidation for 6 h could recover the most colonies.
When the particles were oxidized for 6 h, different amount of antibody (5, 10, 15, 20, 25, 30, 35, 40, 45, 50 µg immunoglobulin per mg of magnetic nanoparticles) were added into the particle suspension (this time the excess antibody was not removed). The proper amount of antibody was defined also by recovery (Table 2). It could be seen from the results that the recovery increased followed by decrease, and had the highest recovery at the antibody of 25 µg/mg nanoparticles.
Five, 10, 15, 20, 25, 30 µL of particle suspension was added into 1 mL samples separately, and then incubated for 30 min followed by 5 min of magnetic separation. The recovery showed that almost the same results could be gained though the amount of particles added was different (Table 3).
Five microliters of particles was added to 1 mL samples, and the mixture was incubated for 5, 10, 15, 20, 30, 40, 50, 60 min. In order to detect the targeted bacteria as fast as possible, 10 min of incubation time was enough to obtain relatively high recovery (Table 4).
The samples were magnetically separated for 1, 2, 3, 4, 5 min (Table 5) adopting the above results from the other factors. Two minutes of separation could draw almost all the particle-bacteria complex to the side wall of the tube, when 1.5 mL eppendorf tube was used and the intensity of magnetic field was 0.23 T.
From the above figures, we could see that the recovery remained at the level of more than 90% when the bacteria suspension was diluted to 102 CFU/mL in water. Further experiments tested that even if there were only 10 bacteria in the samples, the particles still could capture 7-8 organisms. The sensitivity was 101-102 CFU/mL in the presence of 108 CFU/mL of other organisms.
Five microliters of immunomagnetic nanoparticles (specific to E.coli O157:H7) with 1 mL of other bacteria suspension was mixed separately, which included other serotypes of E.coli, S.aureus, Salmonella, Shigella, and Y.enterocolitica. After magnetic separation, the particle-bacteria complex was transferred onto selective plates specific to different strains. As a result, colony of other serotypes of E.coli were not found on the SMAC plates, and at the same time, there was no bacterial growth on their own selective plates. This indicated that the magnetic nanoparticles had high specificity and no cross reactivity and nonspecific binding were observed.
We fetched some foods such as ham, liver, ground beef, rice, steamed bun and milk from local supermarkets. Food samples were prepared according to the reports of some documents[21,22]. A 1-g food samples were added to 0.1 mL of an O157 suspension (101 CFU/mL). After various hours of enrichment culture, 1 mL of samples were detected as above. We gladly found that the target micro-organisms could be isolated from food samples after 2.5 h of enrichment (but for milk, it had to take 4 h), much shorter than regular enrichment time, in the presence of at least 108 CFU/mL background bacteria. Food granules showed little interference with IMS.
Food safety has attracted increased attention of the government and general public. Some microbiological detection technique have been used to identify the infection of gastrointestinal tract[23,24]. Traditional methods for the detection of trace amount of bacteria require amplification or enrichment of the target bacteria in the sample[25-27]. These methods tend to be laborious and time-consuming because of the complicated assay procedures. Strategies based on molecular biology, such as PCR have been made to improve the sensitivity of detection, and gene chip to be adaptable to high-throughput bioanalysis for multiple pathogens. But these strategies are trapped by pre-enrichment and isolation of target bacteria from the samples. In order to shorten the process of enrichment, effective measure of concentration must be taken to sufficiently increase the cell density.
IMS was a technique emerged in the 1980s. Magnetic microspheres (MMS) were a kind of solid particles that had a core of metal ions covered with macromolecule polymer. Attracted by magnetic field, MMS could separate from other components in the liquid. When coupled with specific antibody, they got the ability to capture antigen even when there were a lot of background bacteria. Compared with other isolation methods, IMS was a physical separation that sublethally injured cells might be recovered more efficiently by IMS from foodstuffs and hence reduced the number of false negative results. Magnetic iron-dextran nanoparticles are an improvement over previously prepared MMS; in that they are extremely stable and do not aggregate during mild protein coupling reactions or bacteria isolating procedures. This is largely due to their small size and the substantial dextran coating which surround the iron oxide core.
In this paper, immunomagnetic nanoparticles were used to facilitate the separation and concentration of E.coli O157:H7 from other components in foods. The magnetic iron-dextran nanoparticles are easily synthesized within 30 min, and purified by conventional biochemical separation techniques. In order to isolate target bacteria, we have coupled specific antibody to the nanoparticles, which had been oxidized with periodate under mild conditions. The Schiff’s base linkage produced between an aldehyde group of dextran and an amino group of antibody was further stabilized to a secondary amine by borohydride reduction. Appropriate oxidation time must be confirmed to ensure the final function. If it is short, the amount of the aldehyde group was too small to couple enough antibody. On the other hand, over oxidation would change aldehyde group into carboxyl, and also influence the linkage of antibody. Antibody specificity is also an important condition when using immunomagnetic nanoparticles in positive selection of bacteria. But the amount of antibody should not be too much, because the excess antibody would occupy the antigen site of the bacteria, and the competition between free antibody and combined antibody would occur. As several bacteria might get attached to one particle, papain was used to let antigen detach from antibody. But some particles and bacteria may have been lost in the washing procedure, reducing the number of CFU recovered.
In summary, we have developed a fast and sensitive immunological method for bacteria isolation that uses antibody-conjugated magnetic nanoparticles. The reliable detection of trace amounts of E.coli O157:H7 in inoculated food samples demonstrates the practical usefulness of this method. This technique could be adapted in research using antibodies specific for various bacterial pathogens for the detection of a wide variety of bacterial pathogens. This study clearly exhibits the excellent properties of bioconjugated nanomaterials in applications in bioanalysis and biodetection.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Pardoe H, Chua-anusorn W, St Pierre TG, Dobson J. Detection limits for ferrimagnetic particle concentrations using magnetic resonance imaging based proton transverse relaxation rate measurements. Phys Med Biol. 2003;48:N89-N95. [PubMed] |
| 2. | Cao ZG, Zhou SW, Sun K, Lu XB, Luo G, Liu JH. Preparation and feasibility of superparamagnetic dextran iron oxide nanoparticles as gene carrier. AiZheng. 2004;23:1105-1109. [PubMed] |
| 3. | Okon EE, Pulikan D, Pereverzev AE, Kudriavtsev BN, Zhale P. Toxicity of magnetite-dextran particles: morphological study. Tsitologiia. 2000;42:358-366. [PubMed] |
| 4. | Okon E, Pouliquen D, Okon P, Kovaleva ZV, Stepanova TP, Lavit SG, Kudryavtsev BN, Jallet P. Biodegradation of magnetite dextran nanoparticles in the rat. A histologic and biophysical study. Lab Invest. 1994;71:895-903. [PubMed] |
| 5. | Molday RS, MacKenzie D. Immunospecific ferromagnetic iron-dextran reagents for the labeling and magnetic separation of cells. J Immunol Methods. 1982;52:353-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 309] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Pouliquen D, Le Jeune JJ, Perdrisot R, Ermias A, Jallet P. Iron oxide nanoparticles for use as an MRI contrast agent: pharmacokinetics and metabolism. Magn Reson Imaging. 1991;9:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 152] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Lucet I, Filmon R, Pouliquen D, Le Jeune JJ, Jallet P. Transmission electron microscopy in the structural study of superparamagnetic contrast agents for MRI. Bull Assoc Anat (Nancy). 1994;78:57-59. [PubMed] |
| 8. | Wilson MB, Nakane PK. The covalent coupling of proteins to periodate-oxidized sephadex: a new approach to immunoadsorbent preparation. J Immunol Methods. 1976;12:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Luk JM, Lindberg AA. Rapid and sensitive detection of Salmonella (O: 6,7) by immunomagnetic monoclonal antibody-based assays. J Immunol Methods. 1991;137:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Bonneaux F, Dellacherie E. Fixation of various aldehydic dextrans onto human hemoglobin: study of conjugate stability. J Protein Chem. 1995;14:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Tiefenauer LX, Kühne G, Andres RY. Antibody-magnetite nanoparticles: in vitro characterization of a potential tumor-specific contrast agent for magnetic resonance imaging. Bioconjug Chem. 1993;4:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 95] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Parham N, Spencer J, Taylor D, Ternent H, Innocent G, Mellor D, Roberts M, Williams A. An adapted ImmunoMagnetic cell separation method for use in quantification of Escherichia coli O157: H7 from bovine faeces. J Microbiol Methods. 2003;53:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Bennett AR, MacPhee S, Betts RP. The isolation and detection of Escherichia coli O157 by use of immunomagnetic separation and immunoassay procedures. Lett Appl Microbiol. 1996;22:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Perez JM, Simeone FJ, Saeki Y, Josephson L, Weissleder R. Viral-induced self-assembly of magnetic nanoparticles allows the detection of viral particles in biological media. J Am Chem Soc. 2003;125:10192-10193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 339] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 15. | Jenkins C, Pearce MC, Smith AW, Knight HI, Shaw DJ, Cheasty T, Foster G, Gunn GJ, Dougan G, Smith HR. Detection of Escherichia coli serogroups O26, O103, O111 and O145 from bovine faeces using immunomagnetic separation and PCR/DNA probe techniques. Lett Appl Microbiol. 2003;37:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Hsih HY, Tsen HY. Combination of immunomagnetic separation and polymerase chain reaction for the simultaneous detection of Listeria monocytogenes and Salmonella spp. in food samples. J Food Prot. 2001;64:1744-1750. [PubMed] |
| 17. | Gooding CM, Choudary PV. Rapid and sensitive immunomagnetic separation-polymerase chain reaction method for the detection of Escherichia coli O157: H7 in raw milk and ice-cream. J Dairy Res. 1997;64:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Su XL, Li Y. Quantum dot biolabeling coupled with immunomagnetic separation for detection of Escherichia coli O157: H7. Anal Chem. 2004;76:4806-4810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Lee J, Deininger RA. Detection of E. coli in beach water within 1 hour using immunomagnetic separation and ATP bioluminescence. Luminescence. 2004;19:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Ware MW, Wymer L, Lindquist HD, Schaefer FW. Evaluation of an alternative IMS dissociation procedure for use with Method 1622: detection of Cryptosporidium in water. J Microbiol Methods. 2003;55:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Wright DJ, Chapman PA, Siddons CA. Immunomagnetic separation as a sensitive method for isolating Escherichia coli O157 from food samples. Epidemiol Infect. 1994;113:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 117] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Hara-Kudo Y, Konuma H, Nakagawa H, Kumagai S. Escherichia coli O26 detection from foods using an enrichment procedure and an immunomagnetic separation method. Lett Appl Microbiol. 2000;30:151-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Hunter PR. Drinking water and diarrhoeal disease due to Escherichia coli. J Water Health. 2003;1:65-72. [PubMed] |
| 24. | Deisingh AK, Thompson M. Strategies for the detection of Escherichia coli O157: H7 in foods. J Appl Microbiol. 2004;96:419-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Zanetti F, De Luca G, Morabito S, Sacchetti R, Stampi S. Immunomagnetic assay, classic culture method and fermentation tube test in the recovery of Escherichia coli O157 from sewage. New Microbiol. 2003;26:207-213. [PubMed] |
| 26. | Kerr P, Finlay D, Thomson-Carter F, Ball HJ. A comparison of a monoclonal antibody-based sandwich ELISA and immunomagnetic bead selective enrichment for the detection of Escherichia coli O157 from bovine faeces. J Appl Microbiol. 2001;91:933-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Brovko L, Young D, Griffiths MW. Method for assessment of functional affinity of antibodies for live bacteria. J Microbiol Methods. 2004;58:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |