Published online Jun 28, 2005. doi: 10.3748/wjg.v11.i24.3655
Revised: November 9, 2004
Accepted: December 3, 2004
Published online: June 28, 2005
AIM: To investigate the anticancer effect of a traditional Chinese medicine gambogic acid (GA) in human gastric cancer line BGC-823 and further study the mechanism of apoptosis induction of GA.
METHODS: Low differential human gastric cancer line BGC-823 were treated with GA at different doses and different times, the inhibitory rates were detected by MTT assay. Apoptosis induced by GA in BGC-823 cells was observed by Annexin-V/PI doubling staining flow cytometry assay. And T/C (%) was chosen to detect the inhibition of GA on human gastric adenocarcinoma BGC-823 nude mice xenografts. Apoptosis on nude mice xenografts was observed by Annexin-V/PI doubling staining flow cytometry assay and DNA fragmentation assay. To further determine the molecular mechanism of apoptosis induced by GA, the changes on the expression of bcl-2 and bax genes were detected by RT-PCR.
RESULTS: After incubation with GA, low differential human gastric cancer line BGC-823 was dramatically inhibited in a dose-dependent manner. After these cells were exposed to GA for 24, 48 and 72 h, the IC50 value was 1.02±0.05, 1.41±0.20 and 1.14±0.19 μmol/L, respectively. Apoptosis in BGC-823 cells induced by GA was observed by Annexin-V/PI doubling staining flow cytometry assay. The apoptotic population of BGC-823 cells was about 12.96% and 24.58%, respectively, when cells were incubated with 1.2 μmol/L GA for 48 and 72 h. T/C (%) of human gastric carcinoma adenocarcinoma BGC-823 nude mice xenografts was 44.3, when the nude mice were treated with GA (8 mg/kg). Meanwhile, apoptosis induced by GA was observed in human gastric carcinoma adenocarcinoma BGC-823 nude mice xenografts. The increase of bax gene and the decrease of bc1-2 gene expressions were found by RT-PCR.
CONCLUSION: The inhibition of GA on human gastric cancer line BGC-823 was confirmed. This effect connects with the inducing apoptosis in BGC-823 cells and the molecular mechanism might be related to the reduction of expression of apoptosis-regulated gene bcl-2, and the improvement of the expression of apoptosis-regulated gene bax. The result was also confirmed in vivo.
- Citation: Liu W, Guo QL, You QD, Zhao L, Gu HY, Yuan ST. Anticancer effect and apoptosis induction of gambogic acid in human gastric cancer line BGC-823. World J Gastroenterol 2005; 11(24): 3655-3659
- URL: https://www.wjgnet.com/1007-9327/full/v11/i24/3655.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i24.3655
Gamboge is a dry resin secreted from garcinia hanburryi, and gambogic acid (GA, C38H44O8, mol. wt 628)[1] is the main active compound of gamboge. It is reported in traditional Chinese medicine that gamboge is cold, acidic, acerbic, and poisonous. Gamboge also has effects of detoxification, hemostasis, and as a parasiticide. Early investigations on GA from the 1960s were mainly on the separation and the evaluation of its structure. In the 1980s, the structure, anti-tumor activity in vitro, absorption, distribution and excretion of GA in mice were studied[2]. Later, the anti-tumor activity of gamboge’s crude extract in ethanol in vitro and in vivo, as well as its absorption, distribution and excretion in mice were researched by a Cooperative Gamboge Antitumor Investigation (CGAI)[3,4]. The anticancer activity and the toxicity of the crude extract were also researched clinically[5]. In the 1990s, its activity and components by various separations were investigated by Kong[6]. In 1996, 11 compounds extracted from gamboge were reported by Japanese scholars. Recently, some foreign scientists modified carboxylic to acidamide on GA to discover other active compounds.
Recent observations from our studies in vitro and in vivo indicated that GGA (general gambogic acids) exhibited inhibition on Heps, S180 and EC in Kunming strain of mice[7], on human hepatocellular carcinoma cells SMMC-7721 and BEL-7402, and on human pulmonary carcinoma cell SPC-A1[7,8]. Results from our present study in vitro and in vivo also showed GA had significant inhibition on cultured human gastric carcinoma cells, and could induce apoptosis in these tumor cells. This paper was undertaken to evaluate this inhibition and apoptosis induction of human gastric cancer line BGC-823, and the probable molecular mechanism.
Medicine GA, supplied by the School of Pharmacy in China Pharmaceutical University (Norms: 20 mg/bottle). The sample was dissolved in RPMI-1640 medium (GIBCO, the USA).
Tumor cells BGC-823 cells supplied by the Cell Bank of Shanghai Institute of Cell Biology, were maintained in RPMI-1640 medium supplemented with 10% heated-inactivated calf serum (Sijiqing, Hangzhou, China), benzylpenicillin 100 KIU/L, and streptomycin 100 mg/L, pH 7.4 in an incubator (Hirasawa, Japan) with a humidified atmosphere of 95% air + 50 mL/L CO2 at 37 °C.
Animal Six-week-old athymic nude mice (BALB/c, nu/nu), used for in vivo experiment, were obtained from Shanghai Laboratory Animal Center (SLAC, China). The nude mice were handled according to aseptic techniques and maintained in a specific pathogen-free environment on a 12-h light/12-h dark cycle, with food and water supplied ad libitum. The mice all weighed 18-22 g at the start of the study.
Reagents MTT purchased from Fluka (USA), was dissolved in 0.01 mol/L PBS. PCR primers were synthesized by Sangon Shanghai (China).
Cell growth inhibition 2×104 cells per well were seeded onto a well of 96-well plates for 24 h, treated with various concentrations of GA (100 μL/well), and incubated for 24, 48, and 72 h, respectively. Then, 5 mg/mL MTT solution (20 μL/well) was added to each well, and cells were incubated for an additional 4 h at 37 °C. The supernatant was aspirated, and 100 μL of DMSO were added to the wells to dissolve any precipitate present. The suspension was placed on micro-vibrator for 5 min and the absorbance (A) was then measured at 570 nm by an enzyme immunoassay instrument (DJ-3200, Huadong Electron Tube Company). Cell inhibitory ratio was calculated by the following formula:
Inhibitory ratio (%) = average absorbance of treated group/(1-average absorbance of control group) ×100%
IC50 was calculated by SAS statistical software.
1×106 cells were seeded in 50 mL dishes and incubated for 24 h at 37 °C. Then GA (1.2 μmol/L) was directly added to the dishes and incubated for an additional 24, 48, and 72 h, respectively. Cells were collected, washed with PBS and resuspended in PBS. Apoptotic cell death was identified by double supravital staining with recombinant FITC (fluorescein isothiocyanate)-conjugated Annexin-V and PI, using the Annexin V-FITC Apoptosis Detection kit (Becton Dickinson, the USA) according to manufacturer’s instructions. Flow cytometric analysis was performed immediately after supravital staining. Data acquisition and analysis were performed in a Becton Dickinson FACS Calibur flow cytometer using CellQuest software.
5×106 BGC-823 cells were subcutaneously inoculated into BALB/cA nude mice. The transplantation tumors were ready to use after having been handed down three generations in nude mice. After 12-14 d, the nude mice with implanted tumor were screened for tumor volume. Tumor-bearing mice in which the tumor had reached a volume of about 100-300 mm3 were selected (mice with tumors that are too large or too small were eliminated) and randomly divide them into five groups. The animals should be pair matched such that the median tumor volume for each group is similar. Each therapy group receives the following treatment regimen -0.9% NS (negative control group), 3 mg/kg 5-FU (positive control group) and three dosages of GA (8, 4, and 2 mg/kg). The day of first treatment is set as d 1. Corresponding agent of each group was administrated (iv) thrice per week (the animals in negative group were given 0.9% NS at the same volume) and lasted for 3 wks. Tumor size was measured twice per week in two perpendicular dimensions also with vernier caliper and converted to tumor volume (TV) as the following formula: (a×b2)/2, where a and b refer to the longer and shorter dimensions, respectively. At the same time the animals were weighed twice per week and mortality was monitored during the experimental period to assess toxicity of the treatments. Relative tumor volume (RTV) was calculated according to the equation: RTV = Vt/V0, where V0 is the tumor volume at day 0 and Vt is the tumor volume at day t. And the evaluation index for inhibition was of relative tumor growth ratio T/C=TRTV/CRTV×100%, where TRTV and CRTV represented RTV of treated and Control groups, respectively.
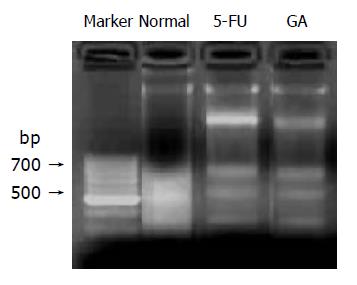
Apoptosis on human gastric adenocarcinoma BGC-823 nude mice xenografts were detected. BALB/cA nude mice were inoculated with BGC-823 cells and fresh tumor tissue was collected after the mice were given GA (8 mg/kg) six times intravenously. The positive drug is 5-FU, with its dose of 25 mg/kg. Early apoptotic cell death was detected by Annexin-V/PI double-staining assay, using the Annexin V-FITC Apoptosis Detection kit (Becton Dickinson, USA) according to manufacturer’s instructions. DNA fragmentation assay was used to exam late apoptosis induced by GA on BGC-823 nude mice xenografts. In this assay, DNA was extracted by Genomic DNA Purification Kit (Fermentas), and then the products were electrophoresed on a 1.5% agarose gel and observed by EB staining using Gel-Pro analyzer.
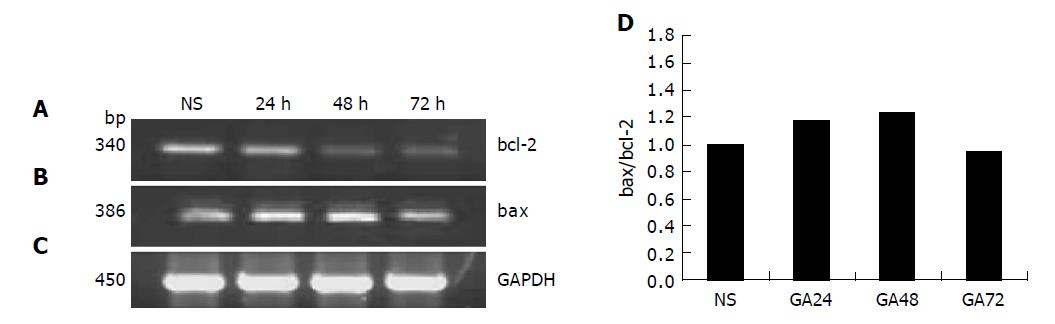
1×106 cells were treated in the presence or absence of 0.8 μmol/L GA for 24, 48, and 72 h, respectively, and total RNA was extracted by TriPure Isolation Reagent (Roche). The primers for Bcl-2, Bax and GADPH were as follows: bcl-2 (340 bp): 5’-TTCCCATCGCTGTCCTTCG-3’, 3’-CGCTTAGATACAAATGTCCGTGTC-5’; bax (386 bp): 5’-GGATGCGTCCACCAAGAA-3’, 3’-AAACACCG-CCCTCACG-5’; GADPH (450 bp): 5’-CTCAGACACCAT-GGGGAAGGTGA-3’, 3’-ATACTGTTGTCGGAGTT-CTAGTA-5’. The following PCR conditions were used: at 94 °C for 5 min, 1 cycle; at 94 °C for 30 s, at 59 °C (bcl-2) or 56 °C (bax) for 30 s, at 72 °C for 45 s, 35 (bcl-2) or 30 (bax) circle; at 72 °C for 7 min, 1 circle. The PCR products were electrophoresed on a 1.5% agarose gel and observed by EB staining using Gel-Pro analyzer.
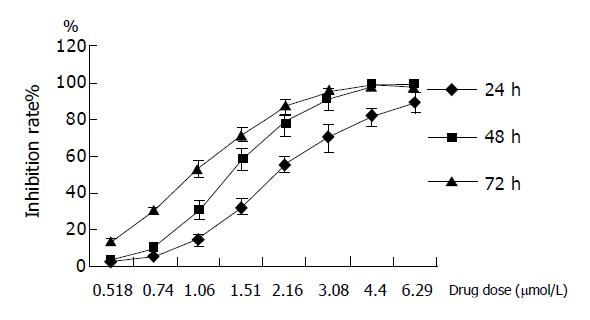
A time- and concentration-dependent inhibition of GA was observed on the low differential human gastric cancer line BGC-823 for 24, 48 and 72 h. IC50 at 24, 48, and 72 h were 2.30±0.26, 1.41±0.15, and 1.02±0.15 μmol/L, respectively. It indicated that when the concentration of GA was lower than 0.74 μmol/L, the inhibition was week; when the concentration ranged from 0.74 to 4.40 μmol/L, the inhibitory effect became obvious, the higher concentration, the better the effect; while the concentration was higher than 4.40 μmol/L, the inhibitory effect was no longer changed. Data of three experiments were shown as mean±SD (Figure 1).
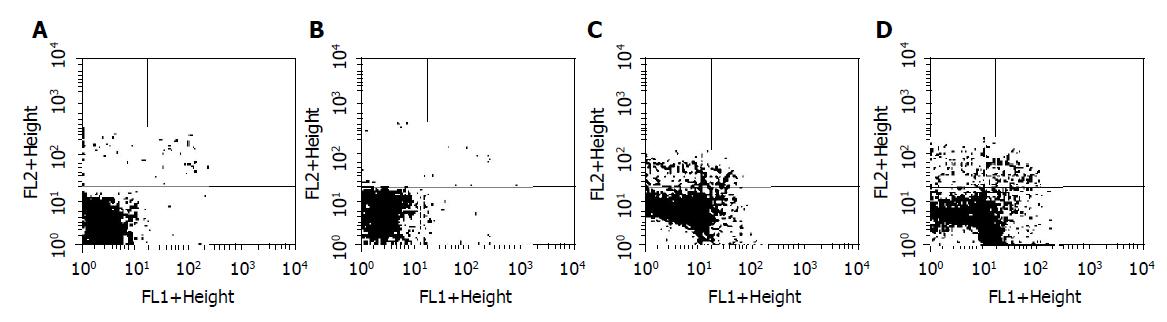
The results of Annexin-V/PI double-staining assay demonstrated that the apoptosis of BGC-823 cells was observed after treatment with 1.2 μmol/L GA for 48 and 72 h, respectively. As shown in Figure 2, untreated cells did not show any significant apoptosis, as well as cells treated with 1.2 μmol/L GA for 24 h, whereas cells were becoming apoptosis after treatment with 1.2 μmol/L GA for 48 and 72 h, with the apoptotic populations of about 12.96% and 24.58%, respectively.
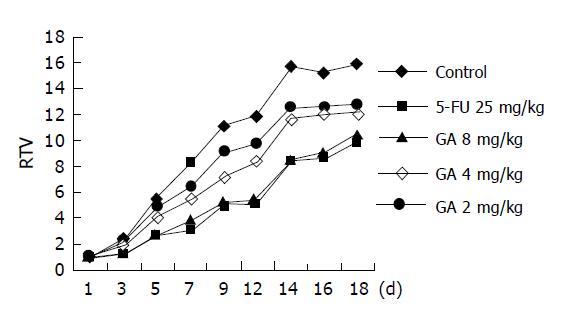
The results showed that GA, as well as 5-FU, was observed to inhibit the growth of BGC-823 nude mice xenografts. The therapeutic effect reached the best on the 12th day after the drug was given to the tested groups first. RTV of the tested group treated with 8 mg/kg GA was 5.2 and T/C (%) was 44.3; RTV of the group treated with 4 mg/kg GA was 8.4 and T/C (%) was 71.1. It suggested that GA (8.4 mg/kg) can distinctly inhibit the growth of transplantation tumor induced by BGC-823 in BALB/cA nude mice (P<0.05), (Table 1 and Figure 3).
| Concentration (mmol/L) | Inhibition rate% | ||
| 24 h | 48 h | 72 h | |
| 0.52 | 2.37±0.45 | 3.88±1.05 | 13.43±2.46 |
| 0.74 | 5.51±0.63 | 10.11±1.89 | 30.05±2.44 |
| 1.06 | 14.50±3.12 | 30.84±4.77 | 53.25±4.65 |
| 1.51 | 32.71±4.23 | 58.49±6.24 | 71.88±3.91 |
| 2.16 | 55.73±4.38 | 78.56±7.18 | 86.57±4.42 |
| 3.08 | 70.44±7.38 | 91.67±5.38 | 94.89±3.80 |
| 4.40 | 81.83±5.08 | 97.80±2.52 | 99.07±0.29 |
| 6.29 | 89.53±5.71 | 97.67±1.94 | 98.54±0.96 |
| IC50 | 2.30±0.26 | 1.41±0.15 | 1.02±0.05 |
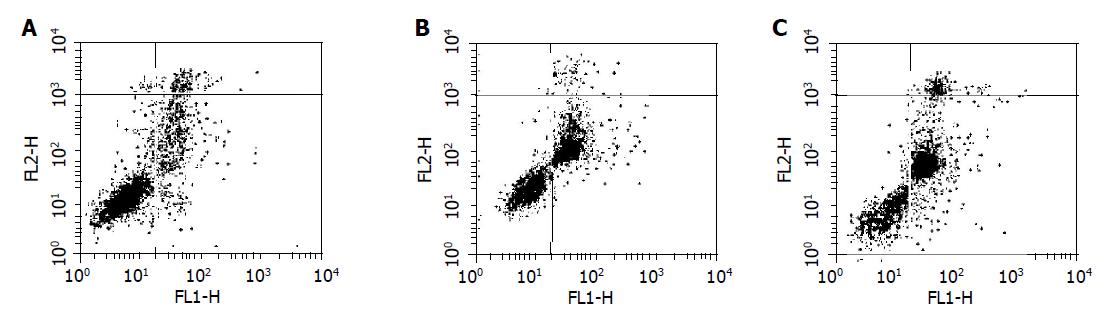
Annexin-V/PI staining assay and DNA fragmentation assay were used to detect the apoptosis inducing by GA on BGC-823 nude mice xenografts. Results by Annexin-V/PI staining assay showed that the early apoptotic population was of about 25% after treatment with GA (8 mg/kg), as shown in Figure 4. At the same time, DNA fragmentation assay provided the evidence of DNA fragmentation of morphological changes of nuclei in BGC-823 cells induced by GA (Figure 5).
It was confirmed that the expression of bcl-2 and bax gene could be regulated by GA. As shown in Figures 6A-6C, after incubation with 0.8 μmol/L GA for 24, 48, and 72 h respectively, the expression of bcl-2 mRNA decreased dramatically, while, on the contrary, the expression of bax mRNA increased. And the value of bax/bcl-2 increased[10,11]. (Figure 6D) The results demonstrated that GA could down-regulate the expression of bcl-2 gene and up-regulate the expression of bax gene. And when the cells were treated with GA (0.8 μmol/L) for 48 h, the regulation was the most distinct.
In recent years, gamboge acid, as a new anticancer drug, has attracted more and more attention, and its effects of anticancer are gradually confirmed, and its work mechanism is still under observations.
In this study, we analyzed effects of GA on proliferation and apoptosis of a human gastric cancer line BGC-823 in vitro and in vivo. The dramatically growth inhibition of GA on BGC-823 cells was observed by MTT assay and the biggest inhibitory rate was 99.07%. The further experiments (Annexin-V/PI double-staining assay) demonstrated that the death was partly caused by the apoptosis induced by GA. In addition, we got the same results by the experiments in vivo. The result of growth inhibition assay on BGC-823 nude mice xenografts demonstrated that the inhibitory effect of GA (8 mg/kg), as well as 5-FU, was dramatically better than the negative control. Apoptosis on human gastric adenocarcinoma BGC-823 nude mice xenografts was detected and the apoptotic population of tumor cells reached 25%, and “DNA ladder” was obvious. So the results confirmed that GA can strongly inhibit the proliferation of human gastric cancer line BGC-823 in vitro and in vivo by inducing apoptosis. To further discuss the exact mechanism of apoptosis induced by GA, we detected the changes on expression of bcl-2 gene and bax gene by RT-PCR. The results showed that GA can down-regulate the expression of bcl-2 mRNA and up-regulate the expression of bax mRNA. It was concluded that inhibitory effect of GA connected with its apoptosis induction by regulation of expression of bcl-2 gene and bax gene.
Apoptosis represents a major protective mechanism against cancer[12], and our observation demonstrated that GA can induce apoptosis on BGC-823 cells. So we can deduce that the high inhibition of GA on BGC-823 cells was partly caused by its apoptosis induction. To further discuss the exact molecular mechanism of apoptosis induced by GA, apoptosis-regulating gene bcl-2 and bax were detected. It is confirmed that bcl-2 gene acts to inhibit apoptosis[13,14], while bax gene induces apoptosis[15,16]. Our results from RT-PCR showed that after treatment with GA (0.8 μmol/L) for 48 h, bcl-2 expression was dramatically decreased and bax expression was slightly increased at mRNA level and the value of bax/bcl-2 increased, thus, apoptosis occurred[17]. We concluded that GA induces apoptosis by up-regulating bax gene and down-regulating bcl-2 gene. And we supposed that the up-regulation of bax gene induced by GA led to the increase of the expression of bax, and the latter not only inhibited the function of bcl-2 in apoptosis by forming heterodimers[11], but also increased the release of cytochrome C[18,19] from the inter membrane space to the cytosol, and then the “intrinsic” pathway[20-23] of apoptosis was activated.
In summary, the anticancer effect of GA may be connected with its ability to regulate the expression of apoptotic-related genes.
Co-first-authors: Wei Liu and Qing-Long Guo
Co-correspondents: Qing-Long Guo
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Lin LJ, Lin LZ, Pezzuto JM, Cordell GA. Isogambogic acid and Isomorellinol from Garcinia Hanburyi. Magn Reson Chem. 1993;31:340-347. |
| 2. | Based on a lecture “The cooperation of Gambogia anticancer investigation”. Presented at JiangXi YiXueYuan XueBao. 1982;3:1-5. |
| 3. | Lei QM, Liu JM, Gong DE. Experimental study on anticancer activity of gamboge. ZhongGuo ZhongLiu ZaZhi. 1985;7:282. |
| 4. | Dong C, Jin TY, Lv FD, Dong RC, Liu JM, Lei QM, Chen BR. In vitro experimental investigation for anticancer effect of gaboge. ZhongGuo YaoLiXue TongBao. 1988;23:89-90. |
| 5. | Sun Z, Jiang XY, Chen WY, Gao WX, Li YF, Tan QM. Some toxicity for injection of gamboge. JiangXi YiYao QiKan. 1983;3:5-7. |
| 6. | Kong LD, Ye DJ, Wu H. General surveys for modern study of gamboge. ZhongHua ZhongYiYao ZaZhi. 1995;20:89-91. |
| 7. | Wu ZQ, Guo QL, You QD, Zhao L. Growth Inhibitory Effect of GGAs on Experimental Tumor in Mice and Huan Tumor Cell Cultured in vitro. ZhongGuo TianRan YaoWu ZaZhi. 2003;1:99-102. |
| 8. | Guo QL, You QD, Wu ZQ, Yuan ST, Zhao L. General gambogic acids inhibited growth of human hepatoma SMMC-7721 cells in vitro and in nude mice. Acta Pharmacol Sin. 2004;25:769-774. [PubMed] |
| 9. | Kelland LR. Of mice and men: values and liabilities of the athymic nude mouse model in anticancer drug development. Eur J Cancer. 2004;40:827-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 248] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Mertens HJ, Heineman MJ, Evers JL. The expression of apoptosis-related proteins Bcl-2 and Ki67 in endometrium of ovulatory menstrual cycles. Gynecol Obstet Invest. 2002;53:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Tilli CM, Stavast-Koey AJ, Ramaekers FC, Neumann HA. Bax expression and growth behavior of basal cell carcinomas. J Cutan Pathol. 2002;29:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Zhang YH, Peng HY, Xia GH, Wang MY, Han Y. Anticancer effect of two diterpenoid compounds isolated from Annona glabra Linn. Acta Pharmacol Sin. 2004;25:937-942. [PubMed] |
| 13. | Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1619] [Cited by in RCA: 1686] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 14. | Pettersson F, Dalgleish AG, Bissonnette RP, Colston KW. Retinoids cause apoptosis in pancreatic cancer cells via activation of RAR-gamma and altered expression of Bcl-2/Bax. Br J Cancer. 2002;87:555-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Heimlich G, McKinnon AD, Bernardo K, Brdiczka D, Reed JC, Kain R, Krönke M, Jürgensmeier JM. Bax-induced cytochrome c release from mitochondria depends on alpha-helices-5 and -6. Biochem J. 2004;378:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Renner S, Weisz J, Krajewski S, Krajewska M, Reed JC, Lichtenstein A. Expression of BAX in plasma cell dyscrasias. Clin Cancer Res. 2000;6:2371-2380. [PubMed] |
| 17. | Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4399] [Cited by in RCA: 4503] [Article Influence: 140.7] [Reference Citation Analysis (0)] |
| 18. | Yamaguchi H, Bhalla K, Wang HG. Bax plays a pivotal role in thapsigargin-induced apoptosis of human colon cancer HCT116 cells by controlling Smac/Diablo and Omi/HtrA2 release from mitochondria. Cancer Res. 2003;63:1483-1489. [PubMed] |
| 19. | Sawa H, Kobayashi T, Mukai K, Zhang W, Shiku H. Bax overexpression enhances cytochrome c release from mitochondria and sensitizes KATOIII gastric cancer cells to chemotherapeutic agent-induced apoptosis. Int J Oncol. 2000;16:745-749. [PubMed] |
| 20. | Reed JC. Apoptosis-regulating proteins as targets for drug discovery. Trends Mol Med. 2001;7:314-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 221] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Wyllie AH. Apoptosis (the 1992 Frank Rose Memorial Lecture). Br J Cancer. 1993;67:205-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 341] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 22. | Niquet J, Wasterlain CG. Bim, Bad, and Bax: a deadly combination in epileptic seizures. J Clin Invest. 2004;113:960-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Liou AK, Clark RS, Henshall DC, Yin XM, Chen J. To die or not to die for neurons in ischemia, traumatic brain injury and epilepsy: a review on the stress-activated signaling pathways and apoptotic pathways. Prog Neurobiol. 2003;69:103-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 216] [Article Influence: 9.8] [Reference Citation Analysis (0)] |