Published online Jun 21, 2005. doi: 10.3748/wjg.v11.i23.3640
Revised: April 23, 2004
Accepted: May 13, 2004
Published online: June 21, 2005
AIM: To detect lymph nodes micrometastases and analyze its correlation with clinicopathological parameters in Dukes’ A and B colorectal cancer patients.
METHODS: One hundred and fourteen patients with colorectal cancer (Dukes’ A 16; Dukes’ B 98) undergoing curative operation without histological lymph nodes metastases were studied between 2001 and 2003. A total of 2 481 lymph nodes were analyzed using monoclonal cytokeratin antibody AE1/AE3 (DAKO, Carpinteria, CA) for immunohist-ochemistry.
RESULTS: In total, 33 (29%) patients were positive for cancer cell by immunohistochemistry. In 31 (94%) patients of them positive nodes showed single tumor cell or small groups of tumor cells; and tumor deposits measuring 0.2 and 0.37 mm in diameter in another 2 (6%) patients. Micrometastases were mainly located in the subcapsular sinus or paracortical sinus. There was no correlation between the positive lymph nodes and gender, age, tumor site, tumor size, histological type, histological grade, invasion depth, Dukes’ staging and microsatellite instability (P>0.05).
CONCLUSION: Our findings suggest that immunohist-ochemical technique using monoclonal cytokeratin antibody AE1/AE3 may be a sensitive and reliable method for detecting lymph nodes micrometastases in Dukes’ A and B colorectal cancer. The clinical significance of lymph nodes microme-tastases is still not confirmed.
- Citation: Zhou ZW, Rieger N, Ruszkiewicz A, Wang GQ, Wan DS. Detection of lymph nodes micrometastases in Dukes’ A and B colorectal cancer using anti-cytokeratin antibodies AE1/AE3. World J Gastroenterol 2005; 11(23): 3640-3643
- URL: https://www.wjgnet.com/1007-9327/full/v11/i23/3640.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i23.3640
Metastasis to regional lymph nodes is an important prognostic factor and is used for the staging of colorectal cancer. It can help clinicians to select the most appropriate treatment for patients. However, even without histological lymph nodes metastases, 20-30% of patients die from a local tumor relapse or distant metastases[1]. This might be explained by the occult cancer metastasis (also termed as micrometastasis) in lymph nodes, which cannot be detected with conventional histological techniques. For example, with routine hematoxylin and eosin (HE) staining sections, lymph node micrometastasis, represented by single cell or small clusters of tumor cells, may be missed. Thus, the application of serial sectioning techniques could provide an improvement, but this procedure is time-consuming. With the immunohistochemical techniques using various antibodies, the possibilities to trace occult tumor cells indicating micrometastatic spread have increased. Therefore, micrometastasis could be detected using these immunohistochemical methods from negative lymph nodes detected with conventional histological techniques. In our study, we aimed to detect lymph nodes micrometastases in Dukes’ A and B colorectal cancer and analyze its correlation with clinicopathological parameters using monoclonal cytokeratin antibody AE1/AE3 in immunohistochemistry; in an effort to find a sensitive and reliable method to detect lymph nodes micrometastases in colorectal cancer patients.
One hundred and fourteen patients with colorectal cancer undergoing curative operation without histological lymph nodes metastases were studied between 2001 and 2003. They included 68 men and 46 women, ranging from 38 to 92 years old, with a median of 71 years. Seventy-eight tumors were located in the colon and 36 in the rectum. According to Dukes’ classification, 16 patients were classified as stage A, 98 patients as stage B. Ninety-eight tumors had adenocarcinoma and 16 had mucous adenocarcinoma. Most patients (92) had a moderately differentiated (G2) carcinoma, 17 had a poorly differentiated carcinoma (G3), and only five patients had a well-differentiated (G1) carcinoma. The mean tumor size was 4.67 cm (ranging from 1.10 to 12.00 cm) in diameter. The microsatellite instability (MSI) was detected in 99 patients, among them 24 were positive, and 75 were negative.
All formalin-fixed and paraffin-embedded lymph nodes were retrieved from the histopathologic archives. A total of 2481 lymph nodes were analyzed. Two serial 3-μm-thick sections were prepared for HE staining and immunohistochemistry. Monoclonal cytokeratin antibody AE1/AE3 (DAKO, Carpinteria, CA) was used for immunohistochemistry. Briefly, sections were deparaffinized, dehydrated, and incubated with AE1/AE3 diluted at 1:200 at room temperature overnight. After incubation with the primary antibody, the slices were washed with phosphate buffered saline (PBS) and then incubated with a rabbit anti-mouse secondary antibody (DAKO, Carpinteria, CA) for 30 min at room temperature. For immunohistologic labeling, the slices were incubated with streptavidin peroxidase for 60 min. The sections were finally counterstained with Mayer’s hematoxylin and mounted. Positive controls consisted of lymph nodes from patients with known metastatic disease; negative controls were performed by omitting the primary antibody.
All slices were examined by two of the investigators and a consensus was reached. Presence of single or group of cytokeratin-positive cells and large tumor deposits (less than 1 mm) was interpreted as positive.
SPSS 11.0 was used for statistical analysis. The χ2 test or Fisher’s exact test was used to examine differences in distribution between groups, the correlation analysis was used to analyze the correlation between the groups. P values of less than 0.05 were considered significant.
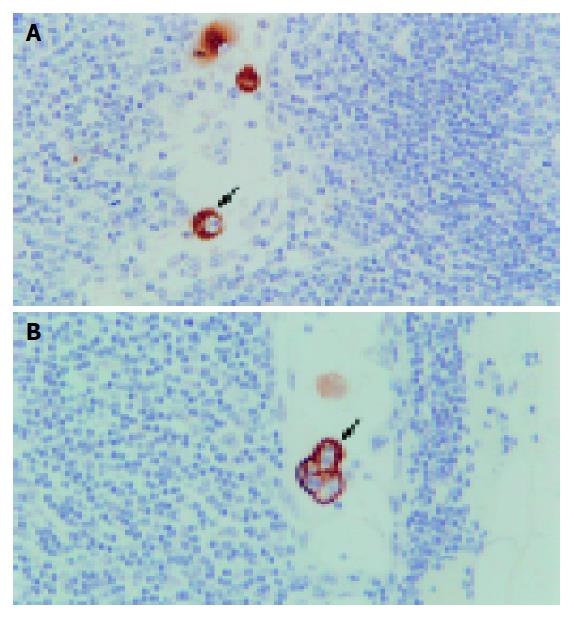
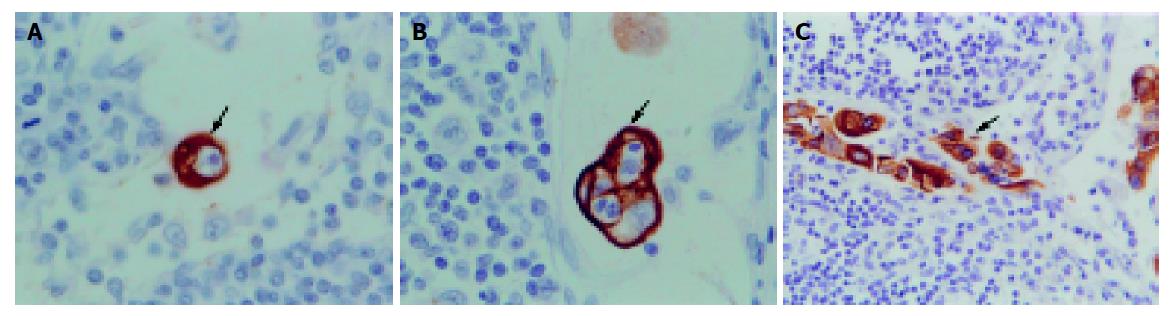
Occult cancer cells were strongly stained with anti-cytokeratin antibody and showed morphological features of malignant cells, such as a large nucleus and condensed nuclear small body. Other components of the lymph nodes were not stained. Stained tumor cells were mainly located in the subcapsular sinus or paracortical sinus (Figure 1). In total, 33 (29%) patients were positive for cancer cells by immunohistochemistry. Among them, positive nodes showed single cells or small groups of cells in 31(94%) patients (Figure 2); positive staining showed tumor deposits measuring 0.2 and 0.37 mm in diameter in two (6%) patients. The total number of positive lymph nodes identified by immunohistochemistry was 52 (2.1%). The average number of positive lymph nodes per case was 1.6. Four (25%) and 29 (29.6%) patients were positive for cancer cells by immunohistochemistry in Dukes’ A and Dukes’ B groups respectively. There was no correlation between the positive lymph nodes and gender, age, tumor site, tumor size, histological type, histological grade, invasion depth, Dukes’ staging and microsatellite instability (all P>0.05).
In the 1970s, single or groups of tumor cells were found in the lymphatic and blood vessel (including the bone marrow) in breast cancer patients. This phenomenon was called occult metastasis or micrometastasis. In 1992, the International Union Against Cancer (UICC)[2] recommended defining the micrometastasis as metastatic single or groups of tumor cells not larger than 2 mm in diameter. Natsugoe et al[3], considered lymph nodes micrometastases to be metastatic single or groups of tumor cells not larger than 0.5 mm. Adell et al[4], described lymph nodes micrometastases to be single cells or groups of tumor cells not more than 100. In most published papers, lymph nodes micrometastases were defined as the metastases detected by immunohistochemical or molecular biological techniques, but not detected in routine stained sections[5-8]. Recently, in 2003, Fisher et al[9], preferred the term mini micrometastases. It comprised single or groups of tumor cells measuring no larger than 1.0 mm, regardless of whether they were detected in routine stained sections, extended pathologic methods such as step or serial sections, or immunohistochemically. Those micrometastases measuring larger than 1.0 mm more accurately represented “missed” or “overlooked” lesions. So far, there has been no uniform standard for lymph nodes micrometastases. We thought the standard brought forward by Fisher et al, was more accurate, and in our study all micrometastases were not larger than 1.0 mm in diameter.
At present, several methods are used to detect lymph nodes micrometastases with their respective advantages and disadvantages. (1) Serial sectioning technique: This method can improve detection of the positive lymph nodes. Gusterson[10] reported that up to 20% of patients who had been diagnosed with lymph nodes negative on routine single section examination could be found to contain micrometastases after serial sections. This procedure is, however, time-consuming and not used routinely. (2) Immunological techniques: These include flow cytometer, radioimmunoassay and immunohist-ochemistry. Immunohistochemistry is a sensitive and commonly used method, and is easy to perform. Many studies[11-13] reported that detection of lymph nodes metastases with immunohisto-chemistry using various antibodies is more sensitive than conventional histological techniques. But the sensitivity of the antibody still needs to be improved. (3) Molecular biological techniques: The reverse transcription-polymerase chain reaction (RT-PCR) technique is often used, which is more sensitive than other methods. Hayashi et al[14], reported that a tumor cell or a mutated cell could be detected by RT-PCR from 106-107 cells. But it can lead to false-positive or false-negative results. Miyake et al[8], revealed that fragmented DNA derived from the main tumor can be detected even in the serum of patients with various types of cancer. Therefore, there is concern that mutated DNA found in the regional lymph nodes might be a fraction of free tumor DNA rather than being derived from cancer cells within the lymph nodes. It only indicates that there are micrometastases or tumor cells in the blood circulation. It is difficult to calculate quantitatively and the cost is high. In our opinion, immunohistochemistry is a more appropriate method to detect lymph nodes micrometastases. In our study, we used monoclonal cytokeratin antibody AE1/AE3 in immunohistochemistry to detect lymph node micro-metastasis in Dukes’ A and B colorectal cancer patients. These patients all underwent curative operation without any discovery of lymph nodes metastases using conventional histological techniques in the resected specimens. The results showed that occult cancer cells were strongly stained with anti-cytokeratin antibody. They were mainly located in the subcapsular sinus or paracortical sinus. In total, 33 (29%) patients were positive for cancer cells by immunohistochemistry. This is consistent with those reported by others, 25-39%[4,11-13], using antibodies directed against various cytokeratins. It indicated that immunohistochemical technique using monoclonal cytokeratin antibody AE1/AE3 is a sensitive and reliable method for detecting lymph nodes micrometastases in Dukes’ A and B colorectal cancer.
As yet, the prognostic relevance of lymph nodes micr-ometastases of colorectal cancer is not really elucidated. Contradicting results are found in the literature[1,4,11-13,15-17]. It might be due to the difference of standards of the micrometastasis and methods of studies. In 2003, Wittekind et al, recommended that metastatic single or groups of tumor cells not larger than 0.2 mm in either lymph nodes or distant sites be classified as N0 and M0, respectively (in other words, as non-metastases) in their book: TNM Supplement-A Commentary on Uniform Use[9]. They might not influence the prognosis of colorectal cancer patients. In our study, in 31 (94%) patients, positive nodes showed single cells or small groups of cells; in 2 (6%) patients, they showed tumor deposits measuring 0.2 and 0.37 mm in diameter. We analyzed the relationship between the lymph nodes micrometastases and clinicopathological parameters in colorectal cancer patients. It showed that the lymph nodes micrometastases detected by immunohistochemistry using monoclonal cytokeratin antibody AE1/AE3 was not correlated with the gender, age, tumor site, tumor size, histological type, histological grade, invasion depth, Dukes’ staging and microsatellite instability (all P>0.05). This indicated that the lymph nodes micrometastases may not influence the prognosis of colorectal cancer patients. Follow up study is required to confirm this.
In conclusion, detection of lymph nodes micrometastases in colorectal cancer patients is still difficult. Immunohis-tochemical technique using monoclonal cytokeratin antibody AE1/AE3 may be a sensitive and reliable method. The prognostic relevance of lymph nodes micrometastases of colorectal cancer is yet to be clarified.
| 1. | Broll R, Schauer V, Schimmelpenning H, Strik M, Woltmann A, Best R, Bruch HP, Duchrow M. Prognostic relevance of occult tumor cells in lymph nodes of colorectal carcinomas: an immunohistochemical study. Dis Colon Rectum. 1997;40:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Hermanek P. Disseminated tumor cells versus micrometastasis: definitions and problems. Anticancer Res. 1999;19:2771-2774. [PubMed] |
| 3. | Natsugoe S, Mueller J, Stein HJ, Feith M, Höfler H, Siewert JR. Micrometastasis and tumor cell microinvolvement of lymph nodes from esophageal squamous cell carcinoma: frequency, associated tumor characteristics, and impact on prognosis. Cancer. 1998;83:858-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Adell G, Boeryd B, Frånlund B, Sjödahl R, Håkansson L. Occurrence and prognostic importance of micrometastases in regional lymph nodes in Dukes' B colorectal carcinoma: an immunohistochemical study. Eur J Surg. 1996;162:637-642. [PubMed] |
| 5. | Occult axillary lymph-node micrometastases in breast cancer. Lancet. 1990;336:434-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Prognostic importance of occult axillary lymph node micrometastases from breast cancers. International (Ludwig) Breast Cancer Study Group. Lancet. 1990;335:1565-1568. [PubMed] |
| 7. | Miyake Y, Fujiwara Y, Ohue M, Yamamoto H, Sugita Y, Tomita N, Sekimoto M, Shiozaki H, Monden M. Quantification of micrometastases in lymph nodes of colorectal cancer using real-time fluorescence polymerase chain reaction. Int J Oncol. 2000;16:289-293. [PubMed] |
| 8. | Miyake Y, Yamamoto H, Fujiwara Y, Ohue M, Sugita Y, Tomita N, Sekimoto M, Matsuura N, Shiozaki H, Monden M. Extensive micrometastases to lymph nodes as a marker for rapid recurrence of colorectal cancer: a study of lymphatic mapping. Clin Cancer Res. 2001;7:1350-1357. [PubMed] |
| 9. | Fisher ER, Colangelo L, Wieand S, Fisher B, Wolmark N. Lack of influence of cytokeratin-positive mini micrometastases in "Negative Node" patients with colorectal cancer: findings from the national surgical adjuvant breast and bowel projects protocols R-01 and C-01. Dis Colon Rectum. 2003;46:1021-105; discussion 1021-105;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Gusterson B. Are micrometastases clinically relevant? Br J Hosp Med. 1992;47:247-248. [PubMed] |
| 11. | Cutait R, Alves VA, Lopes LC, Cutait DE, Borges JL, Singer J, da Silva JH, Goffi FS. Restaging of colorectal cancer based on the identification of lymph node micrometastases through immunoperoxidase staining of CEA and cytokeratins. Dis Colon Rectum. 1991;34:917-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 183] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Greenson JK, Isenhart CE, Rice R, Mojzisik C, Houchens D, Martin EW. Identification of occult micrometastases in pericolic lymph nodes of Duke's B colorectal cancer patients using monoclonal antibodies against cytokeratin and CC49. Correlation with long-term survival. Cancer. 1994;73:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Jeffers MD, O'Dowd GM, Mulcahy H, Stagg M, O'Donoghue DP, Toner M. The prognostic significance of immunohistochemically detected lymph node micrometastases in colorectal carcinoma. J Pathol. 1994;172:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 170] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Hayashi N, Ito I, Yanagisawa A, Kato Y, Nakamori S, Imaoka S, Watanabe H, Ogawa M, Nakamura Y. Genetic diagnosis of lymph-node metastasis in colorectal cancer. Lancet. 1995;345:1257-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 192] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Sasaki M, Watanabe H, Jass JR, Ajioka Y, Kobayashi M, Matsuda K, Hatakeyama K. Occult lymph node metastases detected by cytokeratin immunohistochemistry predict recurrence in "node-negative" colorectal cancer. J Gastroenterol. 1997;32:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Liefers GJ, Cleton-Jansen AM, van de Velde CJ, Hermans J, van Krieken JH, Cornelisse CJ, Tollenaar RA. Micrometastases and survival in stage II colorectal cancer. N Engl J Med. 1998;339:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 407] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 17. | Oberg A, Stenling R, Tavelin B, Lindmark G. Are lymph node micrometastases of any clinical significance in Dukes Stages A and B colorectal cancer? Dis Colon Rectum. 1998;41:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 4.6] [Reference Citation Analysis (0)] |