Published online Jun 21, 2005. doi: 10.3748/wjg.v11.i23.3533
Revised: September 9, 2004
Accepted: October 8, 2004
Published online: June 21, 2005
AIM: To evaluate the in vivo effect of glutamine on cobalt-generated oxidative stress and (HO-1) induction in rat liver.
METHODS: Fasted female Wistar rats received a single injection of cobalt chloride (375 µmol/kg body weight) and then were killed at different times. Lipid peroxidation and soluble and enzymatic antioxidant defense system (reduced glutathione (GSH), catalase (CAT), glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD)) were measured in liver homogenates. Ferritin and ferritin iron contents as well as heme oxygenase-1 (HO-1) activity and expression were also determined. The antioxidant properties of glutamine (Gln) were also evaluated.
RESULTS: Cobalt chloride increased lipid peroxidation (50% over control values) 1 h after treatment. GSH reached a minimum at 3 h (40%) increasing thereafter. Twelve hours after CoCl2 injection, the antioxidant enzymes CAT, GSH-Px and SOD also diminished by about 30%. Heme oxygenase-1 induction was observed 6 h after treatment reaching a maximum value of 14-fold over the controls, 12 h after cobalt treatment. A 1.7-fold increase in ferritin and ferritin-bound iron 24 h after treatment were also obtained. Administration of glutamine (300 mg/kg body weight) by gavage 24 h before CoCl2 treatment entirely prevented the increase in thiobarbituric acid reactive substances (TBARS) content, the decrease in GSH levels, and partially reverted heme oxygenase-1 induction.
CONCLUSION: These results suggested that a natural product such as glutamine prevents glutathione depletion and consequently heme oxygenase induction.
-
Citation: Gonzales S, Polizio AH, Erario MA, Tomaro ML. Glutamine is highly effective in preventing
in vivo cobalt-induced oxidative stress in rat liver. World J Gastroenterol 2005; 11(23): 3533-3538 - URL: https://www.wjgnet.com/1007-9327/full/v11/i23/3533.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i23.3533
Oxidative stress is the result of excessive production of oxidant species and/or depletion of intracellular antioxidant defenses, leading to an imbalance in the redox status of the cell.
Glutamine (Gln) is a multifaceted amino acid used as an energy substrate for most cells[1]; it is also a precursor for nucleotides[2], and it is the most abundant free α-amino acid found in plasma and in the free amino acid pool of the body[3]. One of the most important characteristics of glutamine is that it plays a critical role in glutathione biosynthesis. Glutamine provides glutamate to the glutathione system, which is one of the main sources of the antioxidant defense system in the cell[4,5]. Therefore, Gln is a natural product that plays a leading role in the protection against oxidative stress injury[6,7].
It is accepted that CoCl2 produces oxygen-derived free radicals, which leads to a greater oxidative stress damage[8]. Moreover, it has been demonstrated that cobalt salts activate the expression of several stress-responsive proteins, such as heme oxygenase[9,10].
Heme oxygenase (HO) is the rate-limiting microsomal enzyme that catalyzes heme degradation, which leads to the formation of carbon monoxide, iron and biliverdin, the latter being converted into bilirubin by the cytosolic enzyme biliverdin reductase[11,12]. All these products are biologically active because iron is an important gene regulator[13] and a pro-oxidant[14], bilirubin is a potent antioxidant[15], and CO has properties similar than nitric oxide[16,17]. Three isoforms of HO have been described in mammals: HO-1, the inducible enzyme[18], HO-2, the constitutive isoform[18] and the more recently identified HO-3[19]. HO-1 can be induced in a wide range of animal tissues, particularly liver, following a number of stressful stimuli including its own substrate heme, various heme proteins, heavy metals, glutathione depletion, UVA radiation, hypoxia, hyperoxia, ischemia reperfusion and many others[20-24].
There is compelling evidence that the biological damage attributed to reactive oxygen species (ROS) is dependent on the presence of iron such as heme-derived intracellular iron[25]. Within most cells ferritin constitutes the major storage site for non-metabolized intracellular iron and therefore plays a critical role in regulating the availability of iron to catalyze such harmful reactions as the peroxidation of lipids and the Fenton reaction generating the highly reactive hydroxyl radical (HO·).
Reactive oxygen species occur in tissues and may damage DNA, proteins, carbohydrates, and lipids. These potentially deleterious reactions are controlled by a system of antioxidant defenses which eliminate pro-oxidants and scavenge free radicals. Protection against oxidation is provided by various intracellular compounds such as glutathione, and antioxidant enzymes including catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px)[26].
Previous studies carried out in our laboratory have demonstrated that CoCl2 developed oxidative stress in rat liver and consequently a significant induction of HO-1 enzyme occurred[8]. The present study was performed in order to evaluate the antioxidant properties of glutamine, its capacity to modify glutathione (GSH) levels and its relationship to HO-1.
NADPH, reduced glutathione (GSH), oxidized glutathione (GSSG), 5,5’ dithio-bis-(2-nitrobenzoic acid), thiobarbituric acid, glutathione reductase, ferritin from rat liver, rabbit anti-horse spleen ferritin, 4-chloro-1-naphtol, hydroquinone, 1,10-phenanthroline and glutamine were from Sigma Chemical Company (Saint Louis, MO); peroxidase-conjugated goat anti-rabbit immunoglobulins was from DAKO (Denmark). All other chemicals were of analytical grade.
Animals and treatments Female albino Wistar rats (160-180 g) were housed under standardized conditions with controlled temperature (22±3 °C) and humidity (60%) and exposure to a 12-h light/12-h dark cycle. They were fed regular pelleted rat chow and given tap drinking water ad libitum. Rats were injected subcutaneously with a single dose of cobalt chloride (375 µmol/kg body weight) dissolved in saline solution. Glutamine (300 mg/kg) dissolved in distilled water, was given by gavage 24 h before the cobalt chloride injection. Control animals received saline solution IP and/or distilled water 24 h before the cobalt vehicle. Animals were treated in accordance with guidelines established by the Animal Care and Use Committee of the Argentine Association of Specialists in Laboratory Animals (AADEALC), and were in accordance with the Guide to the Care and Use of Experimental Animals published by the Argentine Council on Animal Care.
Enzyme preparations and assays Rats were anesthetized with sodium pentobarbital (50 mg/kg body weight, intraperitoneally). Then, they were killed by decapitation 1, 3, 6, 9, 12, 15, 18, 24, 28 and 36 h after injection of cobalt chloride. The livers were excised and perfused with an ice-cold saline solution (0.9% NaCl), and then homogenized in a Potter-Elvehjem homogenizer using different solutions. For heme oxygenase assay the homogenate was prepared using 4 V of ice-cold 0.25 mol/L sucrose solution containing 1 mmol/L phenylmethylsulfonyl fluoride, 0.2 mmol/L EDTA and 50 mmol/L potassium phosphate buffer (pH 7.4). Homogenates were centrifuged at 20000 g for 20 min and supernatant fractions centrifuged at 150000 g for 90 min. The microsomal pellet obtained was washed and resuspended in 20 mmol/L potassium phosphate buffer (pH 7.4), containing 135 mmol/L KCl, 1 mmol/L phenylmethylsulfonyl fluoride and 0.2 mmol/L EDTA to a protein concentration of 10 mg/mL. Microsomal HO-1 was obtained from similar procedures as described elsewhere[18]. The 150000 g supernatants obtained from the microsomal preparation were fractionated by addition of ammonium sulfate (AS), and the 40-60% AS fraction dissolved in 10 mmol/L potassium phosphate buffer (pH 7.4) and dialyzed against the same buffer using this preparation as biliverdin reductase. Heme oxygenase activity was determined as described elsewhere[8]. The standard incubation mixture in a final volume of 200 μL contained 10 μmoL potassium phosphate buffer (pH 7.4), 60 nmoL NADPH, 50 μL HO-1 (0.5 mg protein), 50 μL biliverdin reductase (0.42 mg protein), and 200 nmoL hemin. Incubations were carried out at 37 °C during 30 min. Activity was determined by measuring bilirubin formation, which was calculated as the difference in absorbance measured at 455 and 520 nm, employing an ε value of 50 mmol/L·cm (vismax 455 nm)[21]. Superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) activities were determined spectrop-hotometrically in liver homogenates prepared in a medium consisting of 140 mmol/L KCl and 25 mmol/L potassium phosphate buffer (pH 7.4), and centrifuged at 600 r/min for 10 min. The supernatant, a suspension of preserved organelles, was used as homogenate. Catalase activity was determined by measuring the decrease in absorbance at 240 nm[27], glutathione peroxidase activity following NADPH oxidation at 340 nm[28], and superoxide dismutase activity by inhibition of adrenochrome formation rate at 480 nm[29]. One unit in the SOD assay is defined as the amount of enzymatic protein required to inhibit 50% of epinephrine auto-oxidation.
Lipid peroxidation Lipid peroxidation in liver was determined by measuring the rate of production of thiobarbituric acid reactive substances (TBARS), expressed as malondialdehyde equivalents[30]. One volume of homogenate was mixed with 0.5 volume trichloroacetic acid 150 g/L and centrifuged at 2000 r/min for 10 min. The supernatant (1 mL) was mixed with 0.5 mL thiobarbituric acid (0.7 g/L) and boiled for 10 min. After cooling, sample absorbance was read spectrophotometrically at 535 nm. Malondialdehyde concentration was calculated using a ε value of 1.56×105 mol/L·cm.
Endogenous hepatic GSH content Total glutathione (GSH plus GSSG) was determined in liver homogenates after precipitation with 20 mL/L perchloric, and using yeast-glutathione reductase, 5,5’ dithio-bis-(2-nitrobenzoic acid) and NADPH and reading at 340 nm. Oxidized glutathione (GSSG) was determined by the same method in the presence of 2-vinylpyridine. GSH was calculated from the difference between total glutathione and GSSG[31].
Ferritin content determination For ferritin assay the homogenate was prepared using 1 g of tissue in 10 V of ice-cold 10 mmol/L HEPES pH 7.9 solution containing 10 mmol/L KCl and 0.5 mmol/L dithiothreitol. Homogenates were centrifuged at 10000 g for 5 min. Standard horse ferritin diluted in the range of 0.25-15 ng/50 μL Tris-Na (TS) and homogenates (diluted to approximately 50 μg protein/50 μL TS) were applied in triplicate onto nitrocellulose membranes (MSI, Westboro, MA) presoaked in TS using a vacuum dot blot as described by Roskams and Connors[32]. Briefly, membranes were blocked for 1 h at 25 °C with 3 g/L Molico instant non-fat dry milk in TS, rinsed for 5 min thrice with TS and incubated overnight at 4 °C with primary antibody: rabbit anti-horse ferritin. Membranes were then rinsed and incubated with secondary antibody (peroxidase conjugated, goat anti-rabbit immunoglobulins) for 1 h at 25 °C, rinsed again for 5 min three times with TS and developed with a solution containing α-chloronaphthol in methanol and hydrogen peroxide. Blots were quantified by computerized densitometry. Blots were quantified by Gel-Pro® analyzer 3.1 version, Media Cybernetics.
Ferritin iron determination Homogenates were prepared as described above (ferritin content). Ferritin iron levels were determined as following: supernatants were heated at 70 °C for 10 min, centrifuged at 15000 g for 15 min, and resulting supernatants stored at -20 °C. The liver ferritin extract prepared was subjected to acid hydrolysis with 2.8 mol/L HCl at 90 °C for 1 h after which precipitated proteins were removed by centrifugation at 15000 g for 15 min. One milliliter of the resulting supernatant was incubated with 20 μL of 20 g/L hydroquinone and 20 μL of 10 g/L o-phenanthrolene and the optical density at 505 nm was determined. A standard curve was generated based on the absorbance of standard solution of ferrous sulfate at pH 3. As with the liver ferritin extract, the standard iron solution was carried through the acid hydrolysis procedure as well. All glassware for the ferritin iron assay was acid washed and all chemicals and reagents were ultra pure.
Western blot analysis for HO-1 expression Samples of homogenate obtained for HO-1 activity assays were also analyzed by Western immunoblot technique as previously described[33]. An amount of protein (50 µg) from homogenates of control and treated rats was run in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis using a 12% acrylamide resolving gel (Mini Protean II System, BioRad, Hertz, UK). Separated proteins were transferred to nitrocellulose membranes and non-specific binding of antibodies was blocked with 3% non-fat died milk in PBS, pH 7.4 for 1 h at room temperature. Membranes were then probed with polyclonal goat anti HO-1 antibody (Santa Cruz, Bio Tech., CA), (1:300 dilution in Tris-buffered saline, pH 7.4) over night at 4 °C. Immune complexes were detected using donkey anti-goat secondary antibody (1:1500), (Santa Cruz, Bio Tech., California), and were visualized using ECL reagent (Amersham, Pharmacia). Intensity of bands was analyzed with Gel-Pro® analyzer 3.1 version, Media Cybernetics.
Protein determination Protein concentration was evaluated by the method of Lowry et al[34], using bovine serum albumin as standard.
Figures in the text and tables indicate mean±SD. Differences between control and treated were analyzed using the Student’s t-test, taking P<0.05 as significant.
Oxygen reactive species are regarded as initiators of peroxidative cell damage. TBARS measurement was used as an assay for lipid peroxidation in vitro. A significant increase 50±3% in lipid peroxidation was observed 1 h after CoCl2 treatment, reaching a peak 100±5% 3 h later and decreasing thereafter. Control levels were regained 15 h after the injection (Figure 1).
Reduced glutathione is a leading substrate for enzymatic antioxidant functions and is capable of non-enzymatic radical scavenging. It could therefore be expected that if CoCl2 induces the formation of oxidant species, it will also affect GSH-liver levels. Data in Figure 1 showed that GSH concentration in the liver of treated animals decreased by roughly 32±2% in respect to controls, 1 h after CoCl2 treatment. Liver glutathione levels reached a minimum (40±2% of control value) 3 h after injection, increasing thereafter to approach control levels 9 h later. Control animals failed to show any significant changes in the evaluated parameters up to the end of the 36 h observation period (data not shown).
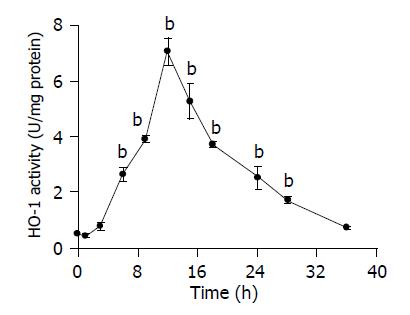
Increased TBARS content as well as GSH decrease appeared as closely related events, taking place several hours before HO-1 induction. Figure 2 shows that HO-1 activity was only evidenced 6 h after CoCl2 administration, with a peak value at 12 h (14-fold over control values), to decrease up to 36 h after treatment, when enzymatic activity was similar to control values.
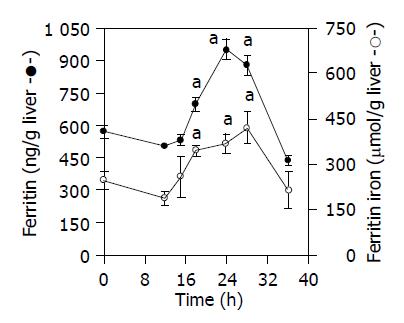
A positive relation between ferritin and liver ferritin iron levels was observed. As shown in Figure 3, the concentration of liver ferritin iron was 40±2% greater than controls 18 h after CoCl2 injection, while a 50±3% increase was found 6 h later, and its concentration remained high up to 28 h after cobalt treatment. Likewise, 18 h after CoCl2 injection, and 12 h after HO induction, ferritin levels were 20±1% higher than those of control animals (Figure 3). An increase by about 67±5% was obtained 24 h after treatment, and this increment was sustained for at least 28 h after CoCl2 administration (Figure 3).
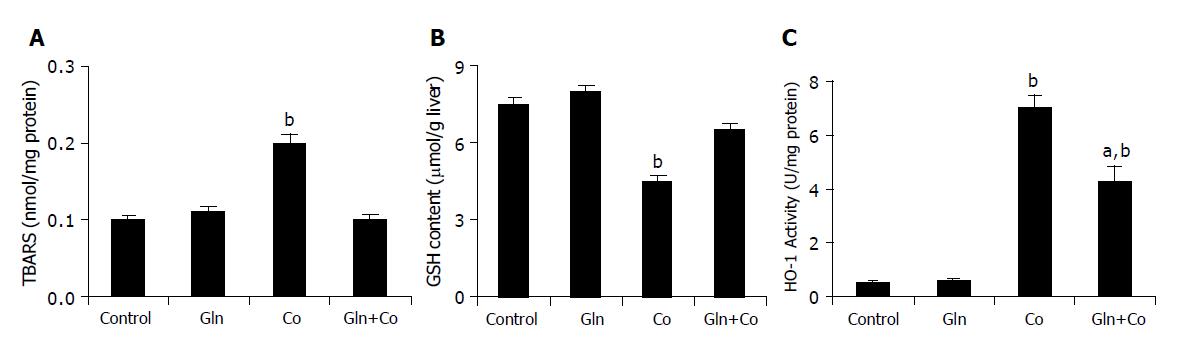
Administration of glutamine 24 h before cobalt treatment entirely prevented TBARS increases as well as GSH decreases, which showed similar levels than control animals (Figures 4A and 4B), and partially prevented the increase in HO-1 activity (Figure 4C). The activity of the antioxidant enzymes CAT, GSH-Px and SOD diminished 12 h after treatment by about 30±5%, compared to control animals, but no effect on antioxidant enzyme activities was observed after Gln pretreatment (Table 1). On the other hand, administration of Gln alone had no effect on HO-1 activity and oxidative stress parameters (Figure 4, Table 1).
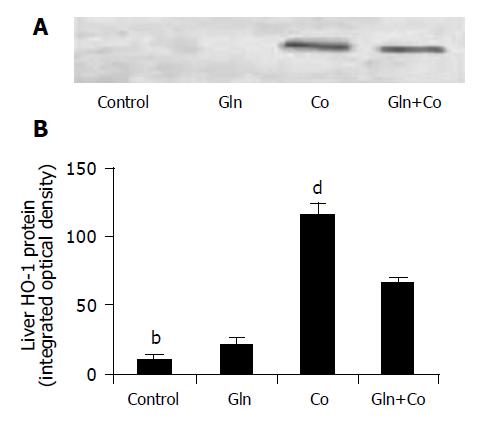
The behavior of HO-1 expression was similar to that observed with HO-1 activity. Therefore, a marked increase in its expression was obtained 12 h after CoCl2 injection, which was partially prevented by Gln administration (Figure 5).
Since the pioneer work of Stocker et al[15], when they demonstrated the antioxidant properties of bilirubin, several authors have proposed that the specific induction of HO-1 by various forms of oxidative stress is part of the defensive mechanism mounted by cells against stress injury.
In agreement, our results clearly demonstrated that after CoCl2 treatment, there is an enhancement in TBARS levels and a decrease in reduced glutathione (GSH) contents. Both events lead to an induction of HO activity (Figures 1 and 2). Depletion of GSH is in fact an index of oxidative stress and it is also linked with the activation of transcriptional factors and regulation of gene expression[35]. Previous works in our laboratory have demonstrated that HO-1 induction occurred once the active oxygen species increased and the antioxidant defense system decreased[8,21]. Moreover, induction of HO-1 has been correlated with a decrease of endogenous GSH[20,22,26].
It is however, worth to note that heme catabolism generates both pro-and antioxidant compounds, consequently influencing cellular sensitivity to oxidants. This issue has been extensively discussed by Ryter and Tyrrell[36]. Several reports have proposed that heme oxygenase induction by various forms of oxidative stress represents an antioxidant defense, operating by decreasing the levels of potential pro-oxidants and increasing the concentrations of active bile pigments, such as bilirubin, capable of acting as antioxidants[8,15]. Accordingly, the higher ferritin levels found in this study, subsequently to HO induction (Figure 3), may be due to HO-dependent release of iron from endogenous heme sources. In this way, there is an enhancement of cellular iron sequestering capacity that may confer increased resistance to oxidative stress. These results are in agreement with recent studies implicating heme oxygenase-dependent increase in ferritin[37,38]. Even though HO is highly induced and therefore bilirubin levels were enhanced, the oxidative stress parameters were still observed. This fact could be due to the pro-oxidant effect of iron exceeds the antioxidant properties of bilirubin. When ferritin increased and therefore iron was subsequently sequestered, the oxidative stress parameters were not detected (Table 1, Figures 1 and 3).
Interestingly, during ROS scavenging, GSH is oxidized and form glutathione-protein mixed disulfides. The cell’s ability to reduce or synthesize GSH (via glutamate) is a key mechanism by which the oxidative stress can be regulated[6]. Besides, it has been demonstrated that Gln preserves gut glutathione levels during intestinal ischemia/reperfusion[39].
Glutamine plays a key role in the protection against oxidative stress injury[6,7]. Our present findings showed that treatment with glutamine, a well known precursor in the GSH biosynthesis, totally reverted the decrease in GSH levels and the increase in lipid peroxidation, and partially inhibited heme oxygenase activity (Figures 4A and 4B). Surprisingly, no effect on antioxidant enzyme activities was found (Table 1). On the other hand, treatment of rats with cobalt produced a significant increase in HO-1 expression, and this effect was also significantly reverted by Gln pretreatment (Figure 5).
Taken together, our data suggest that oxidative stress caused in rat liver the following events: the induction of heme oxygenase produced heme cleavage, which results in increased intracellular free iron, when ferritin content and ferritin-bound iron also beginning to increase, in an attempt to limit iron availability. Induction of ferritin and the concomitant iron sequestration may protect rat liver from oxidative injury by restricting iron-catalyzed free radical reactions. When Gln was administered all these events seemed not to be necessary, to judge by the inhibition in HO-1 activity and expression.
To sum up, our results strongly suggest that the protective effect exerted by Gln was due to the enhancement of GSH, the major soluble antioxidant compound in the liver.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Fox RE, Hopkins IB, Cabacungan ET, Tildon JT. The role of glutamine and other alternate substrates as energy sources in the fetal rat lung type II cell. Pediatr Res. 1996;40:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Boza JJ, Moënnoz D, Bournot CE, Blum S, Zbinden I, Finot PA, Ballèvre O. Role of glutamine on the de novo purine nucleotide synthesis in Caco-2 cells. Eur J Nutr. 2000;39:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Babu R, Eaton S, Drake DP, Spitz L, Pierro A. Glutamine and glutathione counteract the inhibitory effects of mediators of sepsis in neonatal hepatocytes. J Pediatr Surg. 2001;36:282-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Lieth E, LaNoue KF, Berkich DA, Xu B, Ratz M, Taylor C, Hutson SM. Nitrogen shuttling between neurons and glial cells during glutamate synthesis. J Neurochem. 2001;76:1712-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 151] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Dumaswala UJ, Zhuo L, Mahajan S, Nair PN, Shertzer HG, Dibello P, Jacobsen DW. Glutathione protects chemokine-scavenging and antioxidative defense functions in human RBCs. Am J Physiol Cell Physiol. 2001;280:C867-C873. [PubMed] |
| 6. | Matés JM, Pérez-Gómez C, Núñez de Castro I, Asenjo M, Márquez J. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int J Biochem Cell Biol. 2002;34:439-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Mora Lde O, Antunes LM, Francescato HD, Bianchi Mde L. The effects of oral glutamine on cisplatin-induced nephrotoxicity in rats. Pharmacol Res. 2003;47:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Llesuy SF, Tomaro ML. Heme oxygenase and oxidative stress. Evidence of involvement of bilirubin as physiological protector against oxidative damage. Biochim Biophys Acta. 1994;1223:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 226] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Lin JH, Villalon P, Martasek P, Abraham NG. Regulation of heme oxygenase gene expression by cobalt in rat liver and kidney. Eur J Biochem. 1990;192:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Christova TY, Duridanova DB, Setchenska MS. Enhanced heme oxygenase activity increases the antioxidant defense capacity of guinea pig liver upon acute cobalt chloride loading: comparison with rat liver. Comp Biochem Physiol C Toxicol Pharmacol. 2002;131:177-184. [PubMed] |
| 11. | Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1280] [Cited by in RCA: 1349] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 12. | Kutty RK, Maines MD. Purification and characterization of biliverdin reductase from rat liver. J Biol Chem. 1981;256:3956-3962. [PubMed] |
| 13. | Elbirt KK, Bonkovsky HL. Heme oxygenase: recent advances in understanding its regulation and role. Proc Assoc Am Physicians. 1999;111:438-447. [PubMed] |
| 14. | Meneghini R. Iron homeostasis, oxidative stress, and DNA damage. Free Radic Biol Med. 1997;23:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 409] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 15. | Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2595] [Cited by in RCA: 2635] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 16. | Wang R, Wang Z, Wu L. Carbon monoxide-induced vasorelaxation and the underlying mechanisms. Br J Pharmacol. 1997;121:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 231] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1997] [Cited by in RCA: 1976] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 18. | Maines MD, Trakshel GM, Kutty RK. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986;261:411-419. [PubMed] |
| 19. | McCoubrey WK, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997;247:725-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 614] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 20. | Tomaro ML, Frydman J, Frydman RB. Heme oxygenase induction by CoCl2, Co-protoporphyrin IX, phenylhydrazine, and diamide: evidence for oxidative stress involvement. Arch Biochem Biophys. 1991;286:610-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Ossola JO, Tomaro ML. Heme oxygenase induction by cadmium chloride: evidence for oxidative stress involvement. Toxicology. 1995;104:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Ewing JF, Maines MD. Glutathione depletion induces heme oxygenase-1 (HSP32) mRNA and protein in rat brain. J Neurochem. 1993;60:1512-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci USA. 1989;86:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 920] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 24. | Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2000;278:H643-H651. [PubMed] |
| 25. | Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 2nd ed. Oxford: Clarendon Press 1989; 258-296. |
| 26. | Di Mascio P, Murphy ME, Sies H. Antioxidant defense systems: the role of carotenoids, tocopherols, and thiols. Am J Clin Nutr. 1991;53:194S-200S. [PubMed] |
| 27. | Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527-605. [PubMed] |
| 28. | Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3210] [Cited by in RCA: 3381] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 29. | Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170-3175. [PubMed] |
| 30. | Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7899] [Cited by in RCA: 7983] [Article Influence: 169.9] [Reference Citation Analysis (0)] |
| 31. | Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1953] [Cited by in RCA: 1842] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 32. | Roskams AJ, Connor JR. Iron, transferrin, and ferritin in the rat brain during development and aging. J Neurochem. 1994;63:709-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Foresti R, Clark JE, Green CJ, Motterlini R. Thiol compounds interact with nitric oxide in regulating heme oxygenase-1 induction in endothelial cells. Involvement of superoxide and peroxynitrite anions. J Biol Chem. 1997;272:18411-18417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 235] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 34. | Lowry HO, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 35. | Arrigo AP. Gene expression and the thiol redox state. Free Radic Biol Med. 1999;27:936-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 326] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 36. | Ryter SW, Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med. 2000;28:289-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 592] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 37. | Vile GF, Basu-Modak S, Waltner C, Tyrrell RM. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci USA. 1994;91:2607-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 383] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 38. | Gonzales S, Erario MA, Tomaro ML. Heme oxygenase-1 induction and dependent increase in ferritin. A protective antioxidant stratagem in hemin-treated rat brain. Dev Neurosci. 2002;24:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Harward TR, Coe D, Souba WW, Klingman N, Seeger JM. Glutamine preserves gut glutathione levels during intestinal ischemia/reperfusion. J Surg Res. 1994;56:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 3.3] [Reference Citation Analysis (0)] |