Published online Jun 21, 2005. doi: 10.3748/wjg.v11.i23.3514
Revised: December 12, 2003
Accepted: January 29, 2004
Published online: June 21, 2005
AIM: The role of Helicobacter pylori (H pylori ) infection in gastric acid secretion of patients with chronic gastritis remains controversial. This study was designed to elucidate the effect of H pylori on H+/K+-ATPase activities in gastric biopsy specimens.
METHODS: Eighty-two patients with chronic gastritis who had undergone upper endoscopy were included in this study. H pylori infection was confirmed by rapid urease test and histology. Gastric H+/K+-ATPase activities and serum gastrin concentrations were measured by an enzymatic method and radioimmunoassay, respectively. For those patients who received triple therapy for eradicating H pylori, changes in the activity of gastric H+/K+-ATPase and serum gastrin levels were also measured.
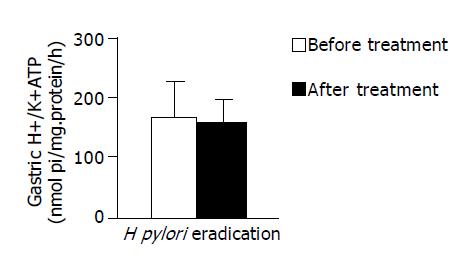
RESULTS: The mean gastric H+/K+-ATPase activity in H pylori-positive group (42 patients) was slightly higher than that in H pylori-negative group (29 patients) (169.65±52.9 and 161.38±43.85 nmol Pi/(mg·h), respectively, P = 0.301). After eradication of H pylori, the gastric H+/K+-ATPase activities slightly decreased compared to prior therapy (165.03±59.50 and 158.42±38.93 nmol Pi/(mg·h), respectively, P = 0.805). The mean basal gastrin concentration was slightly higher in H pylori-positive patients than in H pylori-negative patients (87.92±39.65 pg/mL vs 75.04± 42.57 pg/mL, P = 0.228). The gastrin levels fell significantly after the eradication of H pylori. (Before treatment 87.00±30.78 pg/mL, after treatment 64.73±18.96 pg/mL, P = 0.015).
CONCLUSION: Gastric H+/K+-ATPase activities are not associated with H pylori status in patients with chronic gastritis.
-
Citation: Thong-Ngam D, Tangkijvanich P, Sampatanukul P, Prichakas P, Mahachai V, Tosukowong P. Direct measurement of gastric H+/K+-ATPase activities in patients with or without
Helicobacter pylori- associated chronic gastritis. World J Gastroenterol 2005; 11(23): 3514-3517 - URL: https://www.wjgnet.com/1007-9327/full/v11/i23/3514.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i23.3514
Helicobacter pylori (H pylori ), a Gram-negative spiral-shaped bacterium, has been established as a major etiologic agent of chronic gastritis and peptic ulcer diseases, including duodenal ulcer (DU) and gastric ulcer (GU). The role of H pylori infection in gastric adenocarcinoma and mucosal associated lymphoid tissue lymphoma has also been increasingly recognized[1]. The bacterium predominantly colonizes in mucosa of the gastric antrum where it induces chronic, diffuse and superficial gastritis. Previous studies have demonstrated that H pylori can alter the regulatory mechanisms for gastric acid secretion[2]. Acute infection with the bacterium results in hypochlorhydria, whereas chronic infection may be associated with either hypo- or hyperchlorhydria. In particular, acid secretion tends to be high in patients with DU, but low in those with GU and gastric cancer. Nonetheless, the role of H pylori infection in gastric acid secretion of patients with chronic gastritis remains controversial[3].
Gastric H+/K+-ATPase, an intrinsic membrane protein localized on plasma membranes of the parietal cells, is the key enzyme involved in the final step of acid secretion by catalyzing electroneutral exchange of luminal K+ for cytoplasmic H+ and externally coupled with ATP hydrolysis[4]. This enzyme consists of two subunits designated α- and β-subunits, which are encoded by separate genes. The α-subunit is the catalytic subunit responsible for ion exchange, whereas the β-subunit, which is heavily glycosylated, is necessary for delivery of the α-subunit to plasma membranes[5]. It has been demonstrated that several intracellular signals involving H2 receptors, M3 receptors, and gastrin receptors could stimulate H+/K+-ATPase activities to secrete acid and a decrease in its activities is responsible for a decrease in gastric acid secretion[6]. So far, most studies on gastric acid secretion in patients infected with H pylori were based on the evaluation of basal and stimulated gastric acid output or intragastric pH monitoring[7-10]. To the best of our knowledge, direct measurement of gastric H+/K+-ATPase activities from biopsy specimens has never been performed.
The aim of this study was, therefore, to elucidate the effect of H pylori on gastric H+/K+-ATPase activities in patients with chronic gastritis, which was addressed by measuring gastric H+/K+-ATPase activities in patients with or without H pylori-induced gastritis, and by determining the changes of gastric H+/K+-ATPase activities after eradication of H pylori infections by anti-microbial triple therapy. Moreover, the changes in gastrin concentrations before and after eradication of the bacterium were also analyzed.
Eighty-two patients with chronic gastritis who had undergone upper endoscopy at King Chulalongkorn Memorial Hospital were included in this study. None of these patients had a previous history of gastric surgery, or had taken antibiotics, H2 receptor antagonists, bismuth preparations, or proton pump inhibitors within a month before the study. Endoscopic findings in each patient were recorded and four biopsy specimens (two each from the antrum and the body) were taken for rapid urease test (CLO test, Ballard Medical Products, UT, USA) and histological examination with hematoxylin and eosin and modified Giemsa stain for H pylori. Ten additional mucosal biopsy specimens were taken from the gastric body and immediately stored at -70 °C for further measurement of H+/K+-ATPase activities. Blood samples were also collected after endoscopic examination, and sera were separated promptly by centrifugation and stored at -70 °C until analyzed. All patients gave informed consent to participate in the study. The protocol was approved by the Ethics Committee of Chulalongkorn University.
H pylori infection was defined as either positive by rapid urease test or presence of the bacterium on histological examination by Giemsa staining. For eradication of H pylori, patients were treated with a daily dose of 800 mg ranitidine bismuth citrate, 2 g amoxicillin, and 1 g clarithromycin for 1 wk. Endoscopic examination and gastric mucosal biopsies, as well as blood tests, were performed for these subjects at 4 wk after the therapy. H pylori eradication was defined as negative by rapid urease test and the absence of the bacterium by histological examination.
Gastric membrane vesicles containing high concentrations of H+/K+-ATPase were prepared from gastric biopsy specimens by using a modified method from previously described techniques in pig stomachs[11,12]. In brief, scraped gastric mucosa was minced in buffer E (0.25 mol/L sucrose, 2 mmol/L EDTA, 20 mmol/L Tris-HCl solution; pH 6.8) with a tissue chopper. The minced materials were then homogenized using a Teflon-glass homogenizer. The membrane vesicles were obtained by centrifugation at 100000 g for 120 min. The pellets were then suspended in 5 mmol/L Tris-HCl solution (pH 6.8) to a final suspension of 5 mg protein/mL. H+/K+-ATPase activities in membrane vesicles were measured at 37 °C in 20 mmol/L PIPES-triethanolamine (pH 6.8), as the difference between the amounts of inorganic phosphate (Pi) released from 5’-triphosphate (ATP) in the presence and absence of 10 mmol/L KCl. H+/K+-ATPase activity was expressed as nanomoles Pi released per milliliter of protein per hour.
Plasma gastrin concentrations were measured by radioimm-unoassay (GASK-PR; Biogenetech Co., Ltd).
The data were analyzed by using SPSS for Windows. The descriptive data were presented as mean±SD. The Student’s t test was used to determine the differences in gastric H+/K+-ATPase activities and basal gastrin levels between H pylori-positive and -negative groups. The paired t-test was used to determine whether gastric H+/K+-ATPase activities and serum gastrin levels changed after H pylori eradication. P<0.05 was considered statistically significant.
In this study, there were 35 males and 47 females with their age ranging from 16 to 69 years (mean age, 38.7±13.3 years). H pylori was identified by rapid urease test or Giemsa staining in 50 patients (69%). In the H pylori-positive group, 11 patients were treated with anti-microbial triple therapy and successful eradication of the bacterium was obtained in all of these cases.
The mean gastric H+/K+-ATPase activity in H pylori-positive group was slightly higher than that in H pylori-negative group. However, the difference did not reach any statistical significance (169.65±52.9 and 161.38±43.85 nmol Pi/(mg·h), respectively, P = 0.301, Figure 1). After eradication of H pylori, the gastric H+/K+-ATPase activities were slightly decreased compared to prior therapy (165.03±59.50 and 158.42±38.93 nmol Pi/(mg·h), respectively, P = 0.805, Figure 2).
The mean basal gastrin concentration was slightly higher in H pylori-positive patients than in negative patients but no statistical significance between groups was found (87.92±39.65 pg/mL vs 75.04±42.57 pg/mL, P = 0.228, Figure 3A). However, the basal gastrin levels fell significantly after the eradication of H pylori. The mean basal gastrin concentration measured before treatment (87.00±30.78 pg/mL) was significantly higher than that measured after treatment (64.73±18.96 pg/mL, P = 0.015, Figure 3B).
H pylori infection could produce and exert diverse clinical outcomes in chronically infected patients. In the majority of cases, H pylori caused chronic gastritis unassociated with any serious disease, whereas in others the bacterium might cause peptic ulcer diseases or even gastric cancer[1]. Considerable researches have been conducted on the mechanisms by which H pylori infection leads to different disease entities. In this respect, it has been shown that gastric acid secretory capacity and the distribution of gastritis seem to be important factors. For instance, high acid secretion resulting from antral gastritis could lead to the development of DU, while low acid secretion accompanying corpus gastritis was usually found in patients with GU and gastric cancer[13,14]. Despite these data, the effects of H pylori infection on gastric secretion in patients with chronic gastritis, however, remain controversial. It has been demonstrated that the bacterium can result in increased, decreased, or no overall change in gastric acid secretion among these patients[3]. The different and to some extent, contrasting relationships between gastric acid secretion and H pylori infection in chronic gastritis suggest that all of these data still require further investigation.
Most of the previous studies on gastric acid secretion in patients infected with H pylori were based on the evaluation of basal and stimulated gastric acid output or intragastric pH monitoring[7-10]. In contrast to these conventional methods, the technique used in this study permitted direct measurements of human gastric H+/K+-ATPase activities from biopsy specimens for the first time. This method, indeed, enabled direct investigations of gastric H+/K+-ATPase activities in association with H pylori infection before and after eradication. Following this technique, our data showed that H pylori-positive individuals with chronic gastritis had slightly higher gastric H+/K+-ATPase activities than those without the bacteria, but the difference did not reach any statistical significance. Moreover, the mean gastric H+/K+-ATPase activity after eradication of H pylori infections was comparable to the prior therapy. These results suggested that gastric acid secretion was not associated with H pylori status in patients with chronic gastritis. Nonetheless, a type II error of small samples in our study remained a possible explanation for the unencouraging results. In this respect, it should be noted that gastrin levels were significantly lower after successful eradication of H pylori, which might indicate that H pylori-positive individuals had a propensity of higher gastric acid secretion than those without the bacteria. Nonetheless, evidence provided by the current study needs to be confirmed, since the relationship between gastric acid secretion and H pylori infection is intriguing and worthy of further investigation.
Our finding that eradication of H pylori infection lowered serum gastrin concentrations is consistent with the results of previous reports in patients with DU and normal subjects[8,15]. It has been shown that elimination of H pylori in such patients also lowers serum gastrin levels after a meal or during intravenous infusion of gastrin-releasing peptide[15,16]. However, the mechanism by which eradication of H pylori results in decreased serum gastrin levels remains unclear because it has been shown that the density of antral gastrin or somatostatin cells are not altered after the therapy[17]. Alternatively, it has been suggested that many cytokines derived from H pylori associated gastritis including interleukins (IL)-1β, IL-6 and IL-8, tumor necrosis factor alpha (TNF-α), interferon gamma, and platelet-activating factor, may be responsible for hypergastrinemia, either by augmenting antral G cell function or suppressing antral D cell function[18,19]. Interestingly, H pylori strains bearing the cytotoxin-associated protein A (CagA) were found to induce higher levels of IL-8, IL-1β, IL-6, TNF-α and inflammation in gastric mucosa than CagA-negative strains[20]. Therefore, eradication of H pylori infection may result in the inhibition of these inflammatory cytokines, which in turn contribute to the decrease in serum gastrin levels.
In conclusion, gastric H+/K+-ATPase activities are not associated with H pylori status in patients with chronic gastritis. Mean serum gastrin level can be significantly reduced after the eradication of H pylori infection. However, this change is not accompanied with the alteration of H+/K+-ATPase activities.
| 1. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1907] [Article Influence: 82.9] [Reference Citation Analysis (3)] |
| 2. | Sachs G, Weeks DL, Melchers K, Scott DR. The gastric biology of Helicobacter pylori. Annu Rev Physiol. 2003;65:349-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Talley NJ, Quan C. Review article: Helicobacter pylori and nonulcer dyspepsia. Aliment Pharmacol Ther. 2002;16 Suppl 1:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Shin JM, Besancon M, Bamberg K, Sachs G. Structural aspects of the gastric H,K ATPase. Ann N Y Acad Sci. 1997;834:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Helander HF, Keeling DJ. Cell biology of gastric acid secretion. Baillieres Clin Gastroenterol. 1993;7:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Sachs G, Shin JM, Briving C, Wallmark B, Hersey S. The pharmacology of the gastric acid pump: the H+,K+ ATPase. Annu Rev Pharmacol Toxicol. 1995;35:277-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 251] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Wagner S, Gladziwa U, Haruma K, Varrentrapp M, Gebel M. Effect of Helicobacter pylori infection on 24 hour intragastric acidity in patients with gastritis and duodenal ulcer. Gut. 1992;33:1024-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Feldman M, Cryer B, Lee E. Effects of Helicobacter pylori gastritis on gastric secretion in healthy human beings. Am J Physiol. 1998;274:G1011-G1017. [PubMed] |
| 9. | Furuta T, Baba S, Takashima M, Futami H, Arai H, Kajimura M, Hanai H, Kaneko E. Effect of Helicobacter pylori infection on gastric juice pH. Scand J Gastroenterol. 1998;33:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Taguchi Y, Kaito M, Gabazza EC, Takaji S, Shibata T, Oka S, Ikemura N, Nakao K, Hashimoto Y, Imoto I. Helicobacter pylori inhibits the secretory activity of gastric parietal cells in patients with chronic gastritis. An ultrastructural study. Scand J Gastroenterol. 1997;32:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Maeda M, Tagaya M, Futai M. Modification of gastric (H+ + K+)-ATPase with pyridoxal 5'-phosphate. J Biol Chem. 1988;263:3652-3656. [PubMed] |
| 12. | Beil W, Sewing KF, Busche R, Wagner S. Helicobacter pylori augments the acid inhibitory effect of omeprazole on parietal cells and gastric H(+)/K(+)-ATPase. Gut. 2001;48:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Kaneko H, Konagaya T, Kusugami K. Helicobacter pylori and gut hormones. J Gastroenterol. 2002;37:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Calam J, Gibbons A, Healey ZV, Bliss P, Arebi N. How does Helicobacter pylori cause mucosal damage? Its effect on acid and gastrin physiology. Gastroenterology. 1997;113:S43-S49; discussion S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | el-Omar E, Penman I, Dorrian CA, Ardill JE, McColl KE. Eradicating Helicobacter pylori infection lowers gastrin mediated acid secretion by two thirds in patients with duodenal ulcer. Gut. 1993;34:1060-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 196] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Parente F, Maconi G, Sangaletti O, Minguzzi M, Vago L, Bianchi Porro G. Behaviour of acid secretion, gastrin release, serum pepsinogen I, and gastric emptying of liquids over six months from eradication of helicobacter pylori in duodenal ulcer patients. A controlled study. Gut. 1995;37:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Graham DY. Can therapy even be denied for Helicobacter pylori infection? Gastroenterology. 1997;113:S113-S117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Crabtree JE. Role of cytokines in pathogenesis of Helicobacter pylori-induced mucosal damage. Dig Dis Sci. 1998;43:46S-55S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 139] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Shimada T, Terano A. Chemokine expression in Helicobacter pylori-infected gastric mucosa. J Gastroenterol. 1998;33:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut. 1997;41:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 281] [Article Influence: 10.0] [Reference Citation Analysis (0)] |