Published online Jun 14, 2005. doi: 10.3748/wjg.v11.i22.3479
Revised: June 30, 2004
Accepted: August 5, 2004
Published online: June 14, 2005
AIM: To investigate the mechanism and regulation of differentiation from bone marrow mesenchymal stem cells (MSCs) into hepatocytes and to find a new source of cell types for therapies of hepatic diseases.
METHODS: MSCs were isolated by combining gradient density centrifugation with plastic adherence. The cells were cultured in osteogenic or adipogenic differentiation medium and determined by histochemical staining. MSCs were plated in plastic culture flasks that were not coated with components of extracellular matrix (ECM). When MSCs reached 70% confluence, they were cultured in low glucose Dulbecco’s modified Eagle’s medium supplemented with 10 mL/L fetal bovine serum, 20 ng/mL hepatocyte growth factor (HGF) and 10 ng/mL fibroblast growth factor-4 (FGF-4). The medium was changed every 3 d and stored for albumin, alpha-fetoprotein (AFP) and urea assay. Glycogen store of hepatocytes was determined by periodic acid-Schiff staining.
RESULTS: By combining gradient density centrifugation with plastic adherence, we isolated a homogeneous population of cells from rat bone marrow and differentiated them into osteocytes and adipocytes. When MSCs were cultured with FGF-4 and HGF, approximately 56.6% of cells became small round and epithelioid on d 24 by morphology. Compared with the control, levels of AFP increased significantly from d 12 to 15.5±1.4 µg/L (t = 2.31, P<0.05) in MSCs cultured with FGF-4 and HGF, and were higher (46.2±1.5 µg/L) on d 21 (t = 41.926, P<0.01), then decreased to 24.8±2.2 µg/L on d 24 (t = 10.345, P<0.01). Albumin increased significantly on d 21 (t = 3.325, P<0.01) to 1.4±0.2 µg/mL, and to 2.1±0.7 µg/mL on d 24 (t = 3.646, P<0.01). Urea (2.3±0.4 mmol/L) was first detected on d 21 (t = 6.739, P<0.01), and continued to increase to 2.6±0.9 mmol/L on d 24 (t = 4.753, P<0.01). Glycogen storage was first seen on d 21.
CONCLUSION: The method combining gradient density centrifugation with plastic adherence can isolate MSCs. Rat MSCs may be differentiated into hepatocytes by FGF-4 and HGF. Cytokines may play a more important role in differentiation from rat MSCs into hepatocytes.
-
Citation: Kang XQ, Zang WJ, Song TS, Xu XL, Yu XJ, Li DL, Meng KW, Wu SL, Zhao ZY. Rat bone marrow mesenchymal stem cells differentiate into hepatocytes
in vitro . World J Gastroenterol 2005; 11(22): 3479-3484 - URL: https://www.wjgnet.com/1007-9327/full/v11/i22/3479.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i22.3479
Liver transplant may be the only way to treat patients with a heavily damaged liver. However, enough livers are not available. Moreover, rejection is another problem to be solved. The characteristics and potentials of mesenchymal stem cells (MSCs) suggest that MSCs may be a better way to solve the problems. Bone marrow is a complex tissue containing two kinds of stems cells: hematopoietic stem cells (HSCs) and MSCs. MSCs are nonhematopoietic cells and serve as hematopoietic tissues. It was reported that MSCs could differentiate into mesoderm cells lineage such as osteoblasts[1-3], adipocytes[4-6], neuron and brain cells[7-10], and cardiomyocytes[11-13] in vivo and in vitro. Recent work has convincingly demonstrated that adult bone marrow contains cells capable of differentiating into liver epithelial cells in vivo[14-17]. As far as differentiation from MSCs into hepatocytes in vitro is concerned, Schwartz et al[18], have reported that multipotent adult progenitor cells cultured on matrigel or fibronectin could differentiate into functional hepatocyte-like cells. Fiegel et al[19], cultured HSC on collagen matrix and differentiated them into hepatocytic cells in vitro. Matrigel, fibronectin, and collagen matrix are components of extracellular matrix (ECM).
Up to now, the mechanism of stem cell differentiation is unclear. Most researchers believe that microenvironment play an important role in differentiation of stem cells[20,21]. The differentiation of stem cells is regulated by microenvironment, stem cells have reciprocity with neighboring cells, ECM and cytokines. They have effects on the progress of differentiation of stem cells. It is unknown whether MSCs cultured in plastic culture flasks not coated by ECM can differentiate into hepatocytes in vitro.
Although there are many methods to isolate MSCs from the bone marrow, no optimal method is available. In our experiment, we used a relatively simple method, combining gradient density centrifugation with adherence, to isolate rat MSCs and cultured them in plastic culture flasks not treated by ECM. By induction of acid fibroblast growth factor-4 (FGF-4) and hepatocyte growth factor (HGF), rat MSCs could differentiate into hepatocytes in vitro.
Sprague-Dawley (SD) rats were obtained from Laboratory Animal Center of Xi’an Jiaotong University. Fetal bovine serum (FBS) was purchased from Hangzhou Sijiqing Biological Engineering Material Co. Ltd. Albumin RIA kit and alpha-fetoprotein RIA kit were purchased from Beijing Atom High-tech Co. Ltd. Low glucose Dulbecco’s modified Eagle’s medium containing 1 g/L glucose (DMEM-LG) was purchased from GIBCO. Bovine insulin, 1-methyl-3-isobutylxamthine, indomethacin, dexamethasone and ascorbic acid-2-phosphate were purchased from Sigma-Aldrich. β-Glycerophosphate was purchased from Fulke. Acid FGF-4 and HGF were purchased from R&D Systems, Inc. Plastic cell culture flasks (25 cm2) were purchased from Corning Incorporated (Coster®, USA).
SD rats were killed by knocking at their heads, then put into 750 mL/L alcohol for 15 min. We took the femur, tibia ulna, a total of four bones from one rat. Muscles on the bones were taken out as clean as possible. Medullary cavities of bones were washed with DMEM-LG. The DMEM-LG containing cells were layered over an equal volume of Percoll solution (1.073 g/mL). Mononuclear cells (MNCs) were recovered from the gradient interface and washed with PBS after centrifugation at 900 r/min for 30 min. The MNCs were suspended in 5 mL DMEM-LG supplemented with 100 mL/L FBS, 100 U/mL penicillin, 100 U/mL streptomycin, at last plated in 25-cm2 plastic cell culture flasks at the density of 106/mL. The cells were maintained at 37 °C in 50 mL/L CO2 in fully humidified air. In the following 3 d, the medium was changed every day and then every 3 or 4 d.
Cultured cells were harvested from the culture bottles with 0.25 g/L trypsin. Cultured cells at passage 2 were seeded in six-well cell culture clusters. When the cells grew at 70% confluence, they were still cultured in DMEM-LG supplemented with 100 mL/L FBS (as a control) or treated in osteogenic differentiation medium or adipogenic differentiation medium, respectively. Osteogenic differentiation medium: DMEM-LG supplemented with 100 mL/L FBS, 50 µg/mL ascorbate acid-2-phosphate, 10-8 mol/L dexame-thasone and 10 mmol/L β-glycerophosphate. Adipogenic differentiation medium: DMEM-LG supplemented with 10 mL/L FBS, 1 µmol/L dexamethasone, 10 µg/mL bovine insulin, 0.25 mmol/L 1-methyl-3-isobutylxamthine and 100 µmol/L indomethacin. The medium was changed every 4 d, and cells were used for histochemical staining after the completion of differentiation identified by morphology.
The cultured cells at passage 2 were seeded in the plastic culture flasks at 5×105/bottle. When the cells grew at 70% confluence, the control group was continuously cultured in DMEM-LG supplemented with 10 mL/L FBS, 100 U/mL penicillin, 100 U/mL streptomycin. The hepatocyte differen-tiation group was cultured in DMEM-LG supplemented with 10 mL/L FBS, 20 ng/mL HGF, 10 ng/mL FGF-4, 100 U/mL penicillin and 100 U/mL streptomycin. Each flask was added with 5 mL medium, and changed every 3 d. The medium was stored at -20 °C for albumin, alpha-fetoprotein (AFP) and urea assay.
Concentrations of AFP and albumin in the changed medium were determined by radioimmunoassay on d 3, 6, 9, 12, 15, 18, 21, and 24. It was considered that there might be a very small amount of AFP and albumin in the changed medium. So we condensed the medium five times before radioimmunoassay.
Urea concentrations were determined by colorimetric assay. The control group was used as a negative control, which was cultured in DMEM-LG supplemented with 10 mL/L FBS, 100 U/mL penicillin and 100 U/mL streptomycin. The method could detect the urea at least 10 mmol/L.
In the hepatocyte differentiation group, on days 18, 21, and 24, we took out the medium from the flasks, rinsed cells with PBS thrice, fixed them with 100 mL/L formaldehyde for 30 min. The cells were oxidized in 10 g/L periodic acid for 10 min and rinsed thrice in dH2O. Afterwards, cells were treated with Schiff’s reagent for 10 min, rinsed in dH2O for 10 min, stained with hematoxylin for 2 min, differentiated by 1% alcohol-HCl, and rinsed in dH2O again, observed under an invert microscope.
The data of AFP, albumin and urea were expressed as mean±SE. The results were analyzed by t-test. P<0.05 was considered statistically significant.
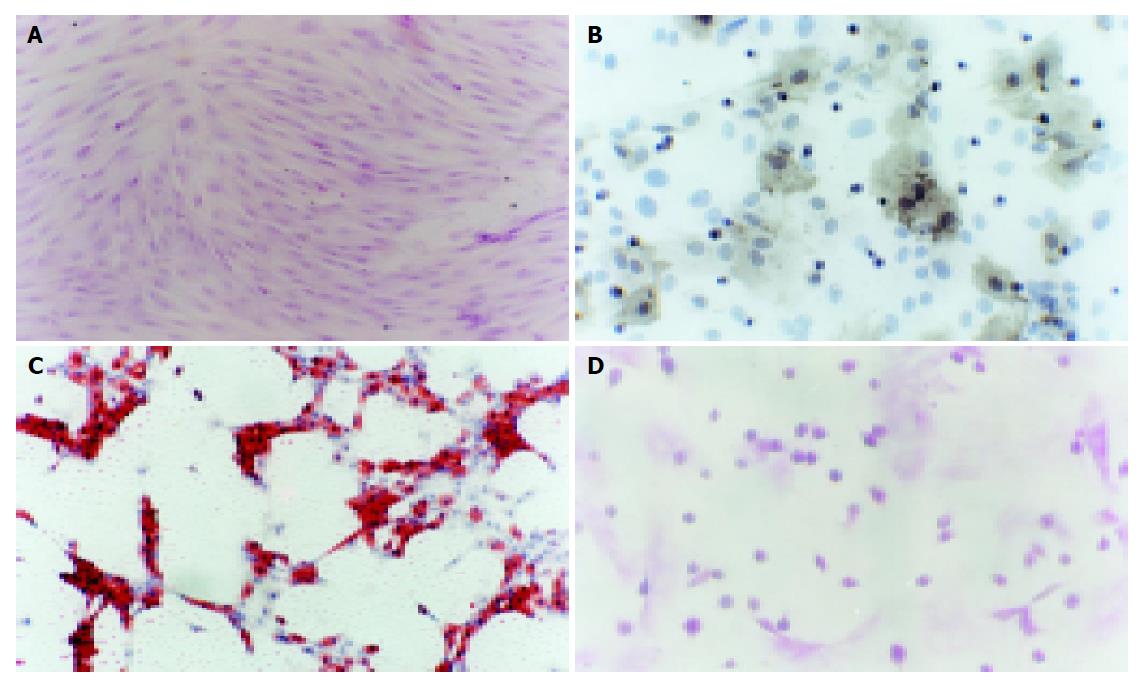
The cells isolated by gradient density centrifugation showed heterogeneity during the first 3 d. The adherent cells were observed on d 1-2. The spindle-shaped cells appeared at the bottom of culture flasks. Many round cells were suspended in the medium. By continuous changes of medium, the suspending cells became less and less. When the medium was changed thrice, the suspending cells were completely removed from the medium. The adhered cells were fibroblast-like and grew as a whirlpool. The sub-cultured cells were much pure and fibroblast-like (Figure 1A). The primary culture cells reached confluence 10-12 d later, the cells sub-cultured at a ratio of 1:3 reached confluence 8-10 d later.
The cells at passage 2 were cultured in DMEM-LG supplemented with 10 mL/L FBS. When the cells grew at 70% confluence, they were treated with or without (control) 50 µg/mL of ascorbate-2 phosphate, 10-8 mol/L of dexamethasone, and 10 mmol/L of β-glycerophosphate. Osteogenic differentiation was attained on d 21 following the treatment. Under the influence of these reagents, approximately 37.5% of the cells formed alkaline phosphatase-positive aggregates (Figure 1B) or von Kossa stain-positive nodules, while the controls did not, suggesting that rat MSCs differentiated into osteocytes. Under the influence of 1 µmol/L dexamethasone, 10 µg/mL bovine insulin, and 0.25 mmol/L 1-methyl-3-isobutylxamthine, the isolated mesenchymal cells formed oil red-O-positive cells on the 3rd d after treatment, while the controls did not. If the lipid droplet appeared in cytoplasm of adipocytes, oil red O would specifically stain them. Six days after treatment, approximately 90.33% of the cells induced adipocytes and reached confluence (Figure 1C).
When the cultured cells reached 70% confluence, they were treated with hepatocyte differentiation medium. The medium contained DMEM-LG supplemented with 10 mL/L FBS, 20 ng/mL HGF, and 10 ng/mL FGF-4, 100 U/mL penicillin and 100 U/mL streptomycin. Before the 6-d treatment they did not show any changes compared with the controls. After treatment, the small round cells appeared in the treatment group, epithelioid cells were also observed. The diameter of the cells was 10-16 µm. On d 24, approximately 56.6% of cells were small round and epithelioid. The control cells were still fibroblast-like, the density of cells was increased. The cells were overlapped in some regions.
By radioimmunoassay, albumin and AFP production was measured at various time points throughout the differentiation. On undifferentiated rat MSCs we could detect low levels of AFP, but not albumin. However, there was a small amount of albumin in the medium. It was hardly detected by albumin RIA assay. Compared with controls, the levels of AFP increased significantly from d 12 with a concentration of 15.5±1.4 µg/L (P<0.05) in MSCs cultured with FGF-4, and HGF continued to increase and was higher on d 21 (P<0.01) with a concentration of 46.2±1.5 µg/L, then decreased to 24.8±2.2 µg/L on d 24 (P<0.01). The level of albumin could not be detected by radioimmunoassay before d 12, albumin increased significantly on d 21 with a concentration of 1.4±0.2 µg/mL (P<0.01), and 2.1±0.7 µg/mL on day 24 (P<0.01). According to the data, increasing time points were different between AFP and albumin (Table 1).
| Group | 3 d | 6 d | 9 d | 12 d | 15 d | 18 d | 21 d | 24 d |
| AFP (µg/L) | ||||||||
| Differentiation | 12.1±1.2 | 11.7±0.8 | 14.4±2.1 | 15.5±1.4a | 24.5±2.9b | 25.9±3.0b | 46.2±1.5b | 24.8±2.2b |
| Control | 11.5±0.9 | 11.6±1.2 | 12.6±2.4 | 13.4±1.9 | 12.8±1.5 | 14.0±0.7 | 12.9±1.3 | 11.1±2.4 |
| Albumin (µg/mL) | ||||||||
| Differentiation | - | - | - | - | 0.5±0.4 | 0.8±0.6 | 1.4±0.2b | 2.1±0.7b |
| Control | - | - | - | - | - | 0.7±0.6 | 0.6±0.5 | 0.6±0.7 |
| Urea (mmol/L) | ||||||||
| Differentiation | - | - | - | - | - | - | 2.3±0.4b | 2.6±0.9b |
| Control | - | - | - | - | - | - | 0.6±0.5 | 0.5±0.5 |
Urea production and secretion by hepatocytes were detected at various time points throughout differentiation. Following treatment with FGF-4 and HGF, urea produced by MSCs was detected with a concentration of 2.3±0.4 mmol/L on d 21, and 2.6±0.9 mmol/L on d 24 (Table 1).
We analyzed the levels of glycogen storage by periodic acid-Schiff (PAS) staining of FGF-4 and HGF induced MSCs on d 18, 21, and 24. Glycogen storage was first seen on d 21, positively stained for PAS (Figure 1D).
In our present study, we used the method combining gradient density centrifugation with plastic adherence to isolate MSCs from rat bone marrow. By the primary induction of multilineage differentiation, the isolated cells satisfied the characteristics of MSCs. Moreover, we cultured rat MSCs in culture flasks not treated by ECM and differentiated them into hepatocytes by induction of HGF and FGF-4, suggesting that cytokines played a most important role in differentiation from rat MSCs into hepatocytes.
There are many methods to isolate bone marrow MSCs from bone marrow, including plastic adherence[22], gradient density centrifugation[23] and immunomagnetic selection[19,24]. Different methods have different defects and virtues. For example, plastic adherence can easily get stem cells on the basis of stem cell plastic adherence characteristics, but it is difficult to get pure stem cells. Gradient density centrifugation depends on the density of MNCs to separate MSCs. Immunomagnetic selection uses the receptors and antigens of MSC. Other methods have also been used to isolate MSCs[25-28], but no one is optimal. In this study we used the Percoll (1.073 g/mL) to isolate MSCs from the bone marrow. After centrifugation, we found many suspended cells in the medium for 72 h. It might be due to the density of cells which was slightly changed in DMEM-LG. So we combined the gradient density centrifugation with plastic adherence and changed the medium thrice to purify MSCs after gradient density centrifugation. According to the results, this method is relatively simple, and can easily get pure MSCs.
MSCs have many differentiating potentials in vivo and in vitro. In order to prove what we have isolated from bone marrow was MSCs, we made the isolated cells differentiate into osteoblasts and adioblasts by induction of reagents. The isolated cells had the characteristics of MSCs, suggesting that the method combining gradient density centrifugation with plastic adherence is an ideal way to isolate rat MSCs.
During the 24 d of differentiation, FGF-4 and HGF induced MSCs into cells with morphological and functional characteristics of hepatocytes. In our study, the cells that were differentiated into hepatocyte-like cells could produce urea, secrete albumin and AFP, and store glycogens. Urea production was characterized by hepatocyte activity, although kidney tubular epithelium also produced urea. In contrast, albumin and AFP production is a specific test for the presence and metabolic activity of hepatocytes. Only hepatocytes can generate and store glycogens. In our research, we found that AFP could be detected throughout the differentiation, because the medium contained a low level of AFP. From d 12, the level of AFP increased significantly compared with controls, suggesting that MSCs began to secrete AFP. It was reported that AFP was produced by immature hepatocytes. That is to say, the hepatocytes were immature on d 12 and 15. Before d 12, the concentration of albumin could not be measured by radioimmunoassay because there was a much small amount of albumin in the medium. When MSCs differentiated, albumin was significantly secreted by MSCs from d 21, and urea was also significantly secreted from d 21. By PAS staining, the differentiated cells could store glycogens. The data suggest that rat MSCs can be differentiated into hepatocytes by induction of FGF-4 and HGF.
Some researchers believe that microenvironment played an important role in differentiation of stem cells[20,21]. The differentiation of stem cells is controlled under microenvironment. Stem cells have reciprocity with adjacent cells, ECM and cytokines. They also have effects on differentiation of stem cells. Schwartz et al[18], and Fiegel et al[19], plated marrow stem cells on matrigel, fibronectin or collagen matrix and differentiated them into hepatocyte-like cells. Matrigel consists of a mixture of ECM components. Fibronectin and collagen matrix are two of ECM components. But in our experiment, we cultured MSCs in plastic culture flasks not treated with matrigel or fibronectin. However, MSCs still differentiated into hepatocytes, suggesting that it is not important to culture MSCs on whatever materials in vitro. Maybe, cytokines play a most important role in differentiating rat MSCs into hepatocytes. It seemed that the ECM could potentially modulate the local concentration of cytokines and cytokines could regulate stem cell proliferation and differentiation. After being cultured with HGF, adult human MSCs could also differentiate into hepatocytes in vitro[19]. HGF was first identified as a blood-derived mitogen for hepatocytes. HGF and its receptor c-Met are the key factors for liver growth and function. FGF-4 is mitogenic for fibroblasts and endothelial cells. Mouse embryonic stem cells grown in medium supplemented with FGF-4 could differentiate into cells expressing hepatocyte-specific genes and antigens[29]. By co-operation of HGF and FGF, the differentiation of MSCs was triggered and MSCs developed into hepatocytes.
MSCs could be isolated, expanded, and maintained in vitro in an undifferentiated state for more than 100 population doublings[30], and differentiate into hepatocytes. The finding may make MSCs as ideal cells for in vivo therapy of genetic or acquired disorders of the liver and for use in bioartificial liver devices. Moreover, MSCs might be used as a new potential therapeutic modality for severe acute pancreatitis. We hope that marrow MSCs will play a more important role in therapy of liver diseases in the future.
| 1. | Chen TL, Shen WJ, Kraemer FB. Human BMP-7/OP-1 induces the growth and differentiation of adipocytes and osteoblasts in bone marrow stromal cell cultures. J Cell Biochem. 2001;82:187-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Ahdjoudj S, Lasmoles F, Oyajobi BO, Lomri A, Delannoy P, Marie PJ. Reciprocal control of osteoblast/chondroblast and osteoblast/adipocyte differentiation of multipotential clonal human marrow stromal F/STRO-1(+) cells. J Cell Biochem. 2001;81:23-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Atmani H, Chappard D, Basle MF. Proliferation and differentiation of osteoblasts and adipocytes in rat bone marrow stromal cell cultures: effects of dexamethasone and calcitriol. J Cell Biochem. 2003;89:364-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Allan EH, Ho PW, Umezawa A, Hata J, Makishima F, Gillespie MT, Martin TJ. Differentiation potential of a mouse bone marrow stromal cell line. J Cell Biochem. 2003;90:158-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Atmani H, Audrain C, Mercier L, Chappard D, Basle MF. Phenotypic effects of continuous or discontinuous treatment with dexamethasone and/or calcitriol on osteoblasts differentiated from rat bone marrow stromal cells. J Cell Biochem. 2002;85:640-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Porter RM, Huckle WR, Goldstein AS. Effect of dexamethasone withdrawal on osteoblastic differentiation of bone marrow stromal cells. J Cell Biochem. 2003;90:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Wislet-Gendebien S, Leprince P, Moonen G, Rogister B. Regulation of neural markers nestin and GFAP expression by cultivated bone marrow stromal cells. J Cell Sci. 2003;116:3295-3302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Song S, Kamath S, Mosquera D, Zigova T, Sanberg P, Vesely DL, Sanchez-Ramos J. Expression of brain natriuretic peptide by human bone marrow stromal cells. Exp Neurol. 2004;185:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Rismanchi N, Floyd CL, Berman RF, Lyeth BG. Cell death and long-term maintenance of neuron-like state after differentiation of rat bone marrow stromal cells: a comparison of protocols. Brain Res. 2003;991:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Lou Sj, Gu P, Chen F, He C, Wang Mw, Lu Cl. The effect of bone marrow stromal cells on neuronal differentiation of mesencephalic neural stem cells in Sprague-Dawley rats. Brain Res. 2003;968:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3891] [Cited by in RCA: 3547] [Article Influence: 147.8] [Reference Citation Analysis (0)] |
| 12. | Orlic D. Adult bone marrow stem cells regenerate myocardium in ischemic heart disease. Ann N Y Acad Sci. 2003;996:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1439] [Cited by in RCA: 1307] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 14. | Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1889] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 15. | Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1764] [Cited by in RCA: 1631] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 16. | Wang X, Montini E, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. Kinetics of liver repopulation after bone marrow transplantation. Am J Pathol. 2002;161:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Mallet VO, Mitchell C, Mezey E, Fabre M, Guidotti JE, Renia L, Coulombel L, Kahn A, Gilgenkrantz H. Bone marrow transplantation in mice leads to a minor population of hepatocytes that can be selectively amplified in vivo. Hepatology. 2002;35:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 663] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 19. | Fiegel HC, Lioznov MV, Cortes-Dericks L, Lange C, Kluth D, Fehse B, Zander AR. Liver-specific gene expression in cultured human hematopoietic stem cells. Stem Cells. 2003;21:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1167] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 21. | Tosh D, Slack JM. How cells change their phenotype. Nat Rev Mol Cell Biol. 2002;3:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 278] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 22. | Meirelles Lda S, Nardi NB. Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br J Haematol. 2003;123:702-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 352] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 23. | Sheng XG, Feng JZ, Wu S, Jin LJ, Yu XY, Zhang B. Differentiation of rabbit bone marrow mesenchymal stem cells into myogenic cells in vitro and expression of vascular endothelial growth factor gene after transfection. DiYi JunYi DaXue XueBao. 2004;24:290-294. [PubMed] |
| 24. | Locatelli F, Corti S, Donadoni C, Guglieri M, Capra F, Strazzer S, Salani S, Del Bo R, Fortunato F, Bordoni A. Neuronal differentiation of murine bone marrow Thy-1- and Sca-1-positive cells. J Hematother Stem Cell Res. 2003;12:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Stamm C, Kleine HD, Westphal B, Petzsch M, Kittner C, Nienaber CA, Freund M, Steinhoff G. CABG and bone marrow stem cell transplantation after myocardial infarction. Thorac Cardiovasc Surg. 2004;52:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Sun S, Guo Z, Xiao X, Liu B, Liu X, Tang PH, Mao N. Isolation of mouse marrow mesenchymal progenitors by a novel and reliable method. Stem Cells. 2003;21:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 27. | Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 211] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C, Deliliers GL. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 424] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 29. | Ruhnke M, Ungefroren H, Zehle G, Bader M, Kremer B, Fändrich F. Long-term culture and differentiation of rat embryonic stem cell-like cells into neuronal, glial, endothelial, and hepatic lineages. Stem Cells. 2003;21:428-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4340] [Cited by in RCA: 3918] [Article Influence: 170.3] [Reference Citation Analysis (0)] |