Published online Jun 14, 2005. doi: 10.3748/wjg.v11.i22.3346
Revised: September 14, 2004
Accepted: October 8, 2004
Published online: June 14, 2005
AIM: To clarify differences in antiviral effect of the drug in patients with different ALT levels, we examined the changes in HBV markers in patients with high or low ALT levels with or without lamivudine treatment.
METHODS: Thirty-seven HBeAg-positive patients were studied. Ten patients with ALT levels higher than 200 IU/L (group 1) and 8 patients with ALT below 200 IU/L (group 2) were treated orally with 100 mg/d of lamivudine. As untreated control, 9 patients with ALT above 200 IU/L (group 3) and 10 patients with ALT below 200 IU/L (group 4) were examined. ALT level, HBeAg/HBeAb status, and HBV DNA level were examined monthly for 11.9±0.4 mo.
RESULTS: The ALT level normalized in all 10 patients of group 1, 7/8 of group 2, 4/9 of group 3, and 1/10 of group 4 within 6 mo (groups 1 vs 2, P = NS; groups 1 vs 3, P = 0.002; groups 1 vs 4, P<0.0001). HBV DNA fell below the detection limit in all 10 patients of group 1, 7/8 of group 2, 0/9 of group 3, and 0/10 of group 4 within 6 mo (groups 1 vs 2, P = NS). HBeAg became seronegative in 7/10 patients of group 1, 1/8 of group 2, 3/9 of group 3, and 0/10 of group 4 within 12 mo (groups 1 vs 2, P = 0.02; groups 1 vs 3, P = NS).
CONCLUSION: Our data suggest that HBeAg-positive patients with higher ALT levels can be considered good candidates for lamivudine therapy, probably because lamivudine accelerates the natural seroconversion of HBeAg, accompanied by HBV DNA loss, in these patients.
- Citation: Kurihara T, Imazeki F, Yokosuka O, Fukai K, Kanda T, Kawai S, Saisho H. Effect of lamivudine in HBeAg-positive chronic hepatitis B: Discordant effect on HBeAg and HBV DNA according to pretreatment ALT level. World J Gastroenterol 2005; 11(22): 3346-3350
- URL: https://www.wjgnet.com/1007-9327/full/v11/i22/3346.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i22.3346
Hepatitis B virus (HBV) infection is a worldwide problem with chronic carriers numbering an estimated 350 million, and with approximately 1.5 million in Japan[1]. Although the natural course of chronic HBV infection is variable, carriers are at risk for developing cirrhosis and hepatocellular carcinoma, and thus need to be followed up and monitored so as to be able to make timely decisions regarding intervention with antiviral therapy[2].
Lamivudine treatment is effective in suppressing HBV replication and decreasing the level of HBV DNA in patients with type B hepatitis[3], thereby improving liver function tests and leading to histological improvement[4-7]. HBeAg seroconversion was found in 17% of patients at 1 year of treatment and in 27% at 2 years[8].
The most troublesome problem of lamivudine treatment is the emergence of lamivudine-resistant strain during the treatment[9-13]. The risk of developing lamivudine resistance increases with the duration of treatment. Reactivation of hepatitis after the cessation of treatment is another problem[14-17]. In this context, knowing the effect of lamivudine on HBV in relation to the clinical status must be of major importance.
The effect of lamivudine was reported to be better in patients with ALT levels elevated to more than 5 times the upper limit of normal (ULN)[18,19]. It is known that such patients tended to seroconvert during interferon treatment[20,21]. Therefore, we studied the effect of lamivudine in relation to the level of ALT, comparing those with and without lamivudine.
The correlations of ALT, HBeAg, and HBV DNA alongside the status of precore and core promoter mutation have not been well demonstrated. We therefore compared the effect of lamivudine in terms of these parameters in a controlled trial.
Between January 2000 and June 2001, 75 patients with HBeAg-positive chronic hepatitis B attended Chiba University Medical Hospital every 1 to 3 mo. Asymptomatic carriers were not included. Eighteen of these patients began treatment with 100 mg/d of oral lamivudine during this period, while the remaining 57 patients chose not to undergo this treatment. Among them, 19 patients were selected as control, and they were not treated with antiviral drug such as lamivudine or interferon throughout the study. There were no patients positive for HCV-Ab, anti-human immuno-deficiency virus antibody or hepatitis D virus antibody, or with a history of drinking over 80 g/d of alcohol. This study was performed in accordance with the Helsinki Declaration.
Thus, a total of 37 patients were classified into 4 groups according to lamivudine treatment and ALT level at enrollment, and they were examined for changes in ALT, HBV DNA and HBeAg/HBeAb. They consisted of 10 treated patients with ALT more than 200 IU/L (group 1), 8 treated patients with ALT less than 200 IU/L (group 2), 9 untreated patients with ALT more than 200 IU/L (group 3), and 10 untreated patients with ALT less than 200 IU/L (group 4) (Table 1). All patients in groups 1 and 2 were continuing lamivudine medication at the time of final observation.
| Group 1 (n = 10) | Group 2 (n = 8) | Group 3 (n = 9) | Group 4 (n = 10) | ||
| Age in years (mean±SD) | 38.8±9.7 | 40.0±9.1 | 34.1±12.9 | 31.2±10.2 | P = 0.07 |
| Gender (male/female) | 10/0 | 6/2 | 4/5 | 7/3 | P = 0.01 |
| ALT (IU/L) | 695±450 | 89±45 | 325±195 | 89±38 | P = 0.01 |
| HBV DNA (LGE/mL) | 7.4±1.4 | 7.5±0.9 | 7.2±1.5 | 8.2±0.5 | P = 0.06 |
| Genotype (A/B/C) | 0/0/10 | 1/0/6 | 0/0/9 | 0/0/10 | P = NS |
| Precore (wild/mutant) | 9/1 | 6/1 | 8/1 | 10/0 | P = NS |
| Core promoter (wild/mutant) | 5/5e | 2/5 | 1/8 | 4/6 | P = 0.14 |
| Fibrosis stage (F1/F2/F3) | 1/3/1 | 3/1/2 | 0/4/1 | 3/1/0 | P = 0.06 |
HBeAg and anti-HBe were examined by ELISA (Abbott Laboratories, Chicago, IL). Anti-HCV was determined by ELISA (Ortho Diagnostics, Tokyo, Japan). Serum HBV DNA was quantified by transcription-mediated amplification (TMA) assay (DNA probe Chugai-HBV, Chugai Diagnostics, Tokyo)[22]. The detection range of this assay was from 3.7 to 8.7-log genome equivalents/mL (LGE/mL).
The G to A mutation at nucleotide (nt) 1 896 in the precore region (A1896 mutation) and the A to T mutation at nt 1762 and G to A mutation at nt 1 764 in the core promoter region (T1762 and A1764 mutations) were determined by the direct sequence method after PCR amplification, basically as previously described[23]. The primers for the first PCR were 5’-TCGCATGGAGACCACCGTGA-3’ (sense, nt 1604-1623) and 5’-ATAGCTTGCCTGAGTGC-3’ (antisense, nt 2076-2060), and the primers for the second PCR were 5’-CATAAGAGGACTCTTGGACT-3’ (sense, nt 1653-1672) and 5’-GGAAAGAAGTCAGAAGGC-3’ (antisense, nt 1974-1957).
HBV genotype was determined from patients’ sera using ELISA (HBV Genotype EIA; Tokushu-Meneki Laboratory, Tokyo, Japan) based on the method described by Usuda et al[24]. Cases in which the HBV genotype could not be determined by this method, we performed PCR of the S-gene region and analyzed the restriction fragment length polymorphism pattern[25].
Proportion of each clinical factor was compared between the groups using the χ2 test and Fisher’s exact probability test, and the group means were compared using the Student’s t-test. The rates of HBV DNA loss, HBeAg loss, and ALT normalization among groups 1 to 4, and those of HBV DNA breakthrough during lamivudine treatment between groups 1 and 2 were analyzed by Kaplan-Meier method, and the difference in incidences was assessed by the log-rank test. The association between HBeAg loss during the follow-up period and clinical factors at baseline including age, sex, HBV-DNA level, the pattern of core promoter or precore mutation, and medication of lamivudine was examined by multivariate Cox regression analysis. P values less than 0.05 were considered significant.
The characteristics of the 37 patients at baseline are shown in Table 1. The mean HBV DNA level was 7.4±1.4 LGE/mL in group 1, 7.5±0.9 in group 2, 7.2±1.5 in group 3, and 8.2±0.5 in group 4 (Table 1). The mean follow-up period was 11.9±0.4 mo (10-12 mo). All the patients had genotype C HBV except one case. Precore mutation was found in 3 patients, one each in groups 1, 2, and 3. Core promoter mutation was found in 50% of group 1, 71% of group 2, 89% of group 3, and 60% of group 4 patients (Table 1).
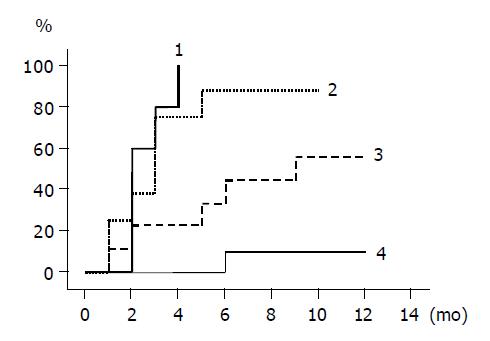
The cumulative incidence of ALT normalization is shown for groups 1 to 4 by Kaplan-Meier method in Figure 1. The rates were different among the groups, and the differences between groups 1 and 3, and groups 1 and 4 were statistically significant (groups 1 and 3, P = 0.002; groups 1 and 4, P<0.0001). The incidence rates between groups 2 and 4, and groups 3 and 4 were also statistically different (groups 2 and 4, P = 0.0002; groups 3 and 4, P = 0.031). In patients given lamivudine, ALT normalized within 6 mo of starting the treatment in all 10 group 1 patients, 7/8 of group 2 patients, while in untreated patients, ALT normalized in 4/9 group 3 patients and remained abnormal in all the group 4 patients except one case within 6 mo.
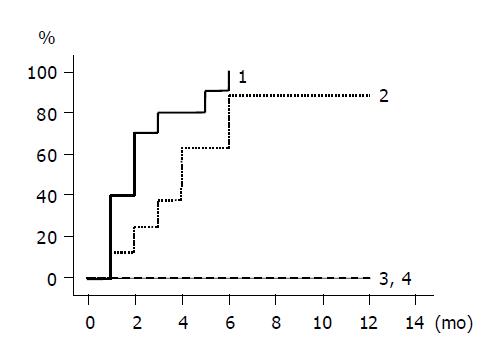
The cumulative incidence of HBV DNA loss is shown by Kaplan-Meier method in Figure 2. Group 1 and 2 patients with lamivudine treatment had higher rates of HBV DNA loss compared to untreated group 3 and 4 patients, whose HBV DNA did not disappear during the follow-up period. HBV DNA levels declined in all the group 1 and 2 patients, falling below the detection limit in all 10 group 1 patients and in 7 of 8 group 2 patients within 6 mo. There was no statistical difference between groups 1 and 2.
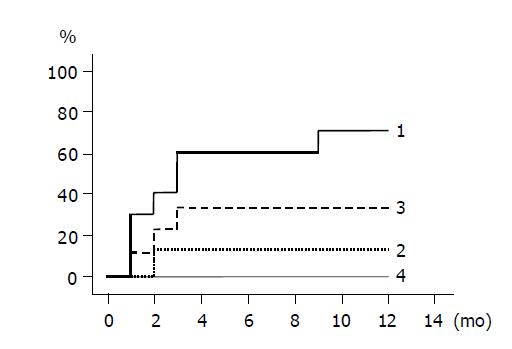
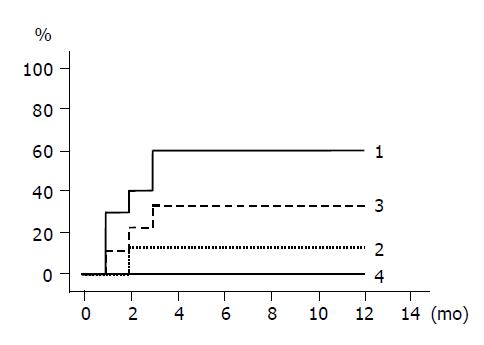
The cumulative incidences of HBeAg loss and HBeAg seroconversion are shown in Figures 3 and 4 respectively. Groups 1 and 3 patients with higher ALT levels had higher rates of HBeAg loss and HBeAg seroconversion compared to groups 2 and 4 patients with lower ALT levels. (Figure 3: groups 1 and 2, P = 0.020; Figure 4: groups 1 and 2, P = 0.048). HBeAg became seronegative in 7 of 10 group 1, 1 of 8 group-2, 3 of 9 group-3, and none of 10 group-4 patients within 12 mo, and became seroconverted in all patients with HBeAg loss except one patient of group 1.
Breakthrough of HBV DNA in spite of continuous lamivudine medication was found in 1 patient of group 1 and in 3 of group 2 during the follow-up period. The cumulative rates of breakthrough at 1 year were 23.5% in the lamivudine-treated patients, 10% in group 1 in contrast to 42.9% in group 2 (P = NS).
HBeAg became negative or seroconverted in 1 of 3 (33%) patients with precore mutation and in 10 of 33 (30%) without precore mutation within 12 mo (P = NS by log-rank test). This result was not affected by lamivudine treatment: 1 of 2 (50%) patients with precore mutation vs 7 of 15 (47%) without precore mutation in those with lamivudine treatment (P = NS by log-rank test), and 0 of 1 (0%) vs 3 of 18 (17%) in untreated patients (P = NS by χ2 test).
HBeAg became negative in 7 of 24 (29%) patients with core promoter mutation and in 4 of 12 (33%) without core promoter mutation within 12 mo (P = NS by log-rank test). HBeAg became negative in 4 of 10 (40%) with core promoter mutation and in 4 of 7 (57%) without mutation in those with lamivudine treatment (P = NS by log-rank test), and in 3 of 14 (21%) with core promoter mutation and in 0 of 5 (0%) without mutation in those untreated (P = NS by χ2 test).
Multivariate Cox regression analysis showed that ALT above 200 IU/L was the only predictive factor with a relative risk of 13.158 associated with HBeAg loss among 7 factors (Table 2).
| Variables | Relative risk | 95% CI | P |
| Age <30 (yr) | 1.0 | ||
| ≥30 (yr) | 1.995 | 0.236-16.898 | 0.526 |
| Gender male | 1.0 | ||
| female | 5.247 | 0.294-93.594 | 0.260 |
| ALT <200 IU/L | 1.0 | ||
| ≥200 IU/L | 13.158 | 1.153-142.857 | 0.038 |
| HBV DNA (LGE/mL) | 0.751 | 0.479-1.178 | 0.212 |
| Core promoter Wild | 1.0 | ||
| Mutant | 0.944 | 0.234-3.817 | 0.936 |
| Precore Wild | 1.0 | ||
| Mutant | 0.749 | 0.050-11.236 | 0.834 |
| Lamivudine Untreated | 1.0 | ||
| Treated | 9.826 | 0.765-126.290 | 0.079 |
Our study has confirmed that the effect of lamivudine on HBe seroconversion is different in patients with ALT more than 200 IU/L and in those with less than 200 IU/L. Although the two groups showed no differences in the rates of HBV DNA disappearance and ALT normalization, the response of HBeAg was quite different between the two groups, with patients with ALT more than 200 IU/L showing a higher seronegative rate than those with a lower level. A higher seroconversion rate for HBeAg was obtained in patients with higher ALT in the treatment with interferon[20,21]. Recently, Chien et al[18], reported that, in lamivudine treatment, HBeAg seroconversion rates were also higher in patients with ALT levels greater than 5 times the ULN compared with those less than this level (7/11, 64% vs 15/129, 12%), results compatible with those of the current study (6/10, 60% vs 1/8, 13%).
The state of HBeAg would be decided by the relative amounts of HBeAg and HBeAb. The production of HBeAg is influenced by HBV replication, precore mutation and/or basic core promoter mutation of HBV. Using a duck hepatitis B virus model, we already reported that both precore wild and mutant viruses reduced their viral DNA levels during lamivudine treatment[26]. As the presence of precore or basic core promoter mutant viruses did not affect the reduction of HBV DNA levels in the sera in the current study, the anti-HBe antibody production level might be the key factor for HBeAg loss and seroconversion.
Patients in untreated groups and with ALT more than 200 IU/L also showed higher rates of HBeAg seroconversion compared to those with ALT less than 200 IU/L in the present study. Liaw reported previously that patient with ALT levels less than and greater than 5 times the ULN have significantly different spontaneous HBeAg seroconversion rates[27]. Because hepatic injuries in patients with chronic hepatitis B are the consequence of cytotoxic T-cell mediated immune hepatocytolysis, higher ALT levels reflect stronger immunological attack of the host against infected HBV. Thus, it seems that patients with high ALT levels tended to have higher rates of HBeAg seroconversion without lamivudine treatment, and the seroconversion rate did not differ between treated (group 1) and untreated (group 3) patients with high ALT levels (Figure 4). However, the rates of ALT normalization and HBV DNA disappearance were quite different between the two groups, with higher rates of ALT normalization and HBV DNA disappearance observed in group 1. Therefore, lamivudine treatment plays a beneficial role in accelerating and maintaining the loss of HBeAg by inhibiting HBV replication in those patients with high ALT.
Our analysis revealed that patients with precore mutation showed no higher rates of HBeAg loss or seroconversion than those without it. Therefore, precore mutation might not be associated with HBeAg loss or seroconversion in lamivudine treatment, although the small number of patients with precore mutation at baseline, only three, and the total number of cases analyzed were too few to draw a definite conclusion. Kuwahara et al[28], reported that eight patients among 12 who lost HBeAg during lamivudine treatment still had precore wild strain, and precore mutant strain reverted to wild in 11 of 17 patients during lamivudine treatment. Maruyama et al[29], also reported that the emergence of precore mutant was a separate event from HBeAg seroconversion.
As for core promoter mutation, Asahina et al[30], reported it to be an independent predictive factor for HBeAg loss during lamivudine therapy in 60 patients with genotype C. However, we could not extract core promoter mutation as a predictive factor by multivariate regression analysis in the current study. This result applied not only to the total patients but also to those treated or untreated with lamivudine, although clinical features at baseline were slightly different between lamivudine-treated and untreated groups in the present study. Further studies will be needed for clarification on this issue.
The reported reappearance of HBV DNA in sera and ALT elevation with the emergence of YMDD mutant of HBV during lamivudine treatment, together with the reactivation of HBV replication and flare-up of hepatitis after cessation of the treatment, are important problems indeed[9-13]. In the current study, patients with ALT more than 200 IU/L showed not only a higher rate of HBeAg seroconversion but also a lower incidence of breakthrough of HBV DNA compared to those with ALT less than 200 IU/L during lamivudine treatment. This implies that patients with ALT more than 200 IU/L can be considered good candidates for the treatment with lamivudine.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1712] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 2. | Lok AS, McMahon BJ. Chronic hepatitis B: update of recommendations. Hepatology. 2004;39:857-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 365] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 3. | Dienstag JL, Perrillo RP, Schiff ER, Bartholomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 620] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 4. | Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, Crowther L, Condreay LD, Woessner M, Rubin M. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1008] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 5. | Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1381] [Cited by in RCA: 1347] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 6. | Suzuki Y, Kumada H, Ikeda K, Chayama K, Arase Y, Saitoh S, Tsubota A, Kobayashi M, Koike M, Ogawa N. Histological changes in liver biopsies after one year of lamivudine treatment in patients with chronic hepatitis B infection. J Hepatol. 1999;30:743-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 574] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 8. | Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Chien RN, Dent J, Roman L, Edmundson S. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology. 2000;119:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 519] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 9. | Seta T, Yokosuka O, Imazeki F, Tagawa M, Saisho H. Emergence of YMDD motif mutants of hepatitis B virus during lamivudine treatment of immunocompetent type B hepatitis patients. J Med Virol. 2000;60:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Nafa S, Ahmed S, Tavan D, Pichoud C, Berby F, Stuyver L, Johnson M, Merle P, Abidi H, Trépo C. Early detection of viral resistance by determination of hepatitis B virus polymerase mutations in patients treated by lamivudine for chronic hepatitis B. Hepatology. 2000;32:1078-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 166] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Liu CJ, Chen PJ, Lai MY, Kao JH, Chen DS. Hepatitis B virus variants in patients receiving lamivudine treatment with breakthrough hepatitis evaluated by serial viral loads and full-length viral sequences. Hepatology. 2001;34:583-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1740] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 13. | Chang TT, Lai CL, Chien RN, Guan R, Lim SG, Lee CM, Ng KY, Nicholls GJ, Dent JC, Leung NW. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2004;19:1276-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Song BC, Suh DJ, Lee HC, Chung YH, Lee YS. Hepatitis B e antigen seroconversion after lamivudine therapy is not durable in patients with chronic hepatitis B in Korea. Hepatology. 2000;32:803-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 257] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Lau DT, Khokhar MF, Doo E, Ghany MG, Herion D, Park Y, Kleiner DE, Schmid P, Condreay LD, Gauthier J. Long-term therapy of chronic hepatitis B with lamivudine. Hepatology. 2000;32:828-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 270] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Leung NW, Lai CL, Chang TT, Guan R, Lee CM, Ng KY, Lim SG, Wu PC, Dent JC, Edmundson S. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology. 2001;33:1527-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 487] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 17. | Ryu SH, Chung YH, Choi MH, Kim JA, Shin JW, Jang MK, Park NH, Lee HC, Lee YS, Suh DJ. Long-term additional lamivudine therapy enhances durability of lamivudine-induced HBeAg loss: a prospective study. J Hepatol. 2003;39:614-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Chien RN, Liaw YF, Atkins M. Pretherapy alanine transaminase level as a determinant for hepatitis B e antigen seroconversion during lamivudine therapy in patients with chronic hepatitis B. Asian Hepatitis Lamivudine Trial Group. Hepatology. 1999;30:770-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 189] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Perrillo RP, Lai CL, Liaw YF, Dienstag JL, Schiff ER, Schalm SW, Heathcote EJ, Brown NA, Atkins M, Woessner M. Predictors of HBeAg loss after lamivudine treatment for chronic hepatitis B. Hepatology. 2002;36:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 226] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Lok AS, Ghany MG, Watson G, Ayola B. Predictive value of aminotransferase and hepatitis B virus DNA levels on response to interferon therapy for chronic hepatitis B. J Viral Hepat. 1998;5:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Wong DK, Cheung AM, O'Rourke K, Naylor CD, Detsky AS, Heathcote J. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. A meta-analysis. Ann Intern Med. 1993;119:312-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 705] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 22. | Kamisango K, Kamogawa C, Sumi M, Goto S, Hirao A, Gonzales F, Yasuda K, Iino S. Quantitative detection of hepatitis B virus by transcription-mediated amplification and hybridization protection assay. J Clin Microbiol. 1999;37:310-314. [PubMed] |
| 23. | Sumi H, Yokosuka O, Seki N, Arai M, Imazeki F, Kurihara T, Kanda T, Fukai K, Kato M, Saisho H. Influence of hepatitis B virus genotypes on the progression of chronic type B liver disease. Hepatology. 2003;37:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 314] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 24. | Usuda S, Okamoto H, Iwanari H, Baba K, Tsuda F, Miyakawa Y, Mayumi M. Serological detection of hepatitis B virus genotypes by ELISA with monoclonal antibodies to type-specific epitopes in the preS2-region product. J Virol Methods. 1999;80:97-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 224] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 25. | Mizokami M, Nakano T, Orito E, Tanaka Y, Sakugawa H, Mukaide M, Robertson BH. Hepatitis B virus genotype assignment using restriction fragment length polymorphism patterns. FEBS Lett. 1999;450:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 183] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Tomita T, Yokosuka O, Tagawa M, Saisho H, Tamura S, Fukuda I, Omata M. Decrease of wild-type and precore mutant duck hepatitis B virus replication during lamivudine treatment in white Pekin ducks infected with the viruses. J Hepatol. 2000;32:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Liaw YF. Hepatitis flares and hepatitis B e antigen seroconversion: implication in anti-hepatitis B virus therapy. J Gastroenterol Hepatol. 2003;18:246-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Kuwahara R, Kumashiro R, Murashima S, Ogata K, Tanaka K, Hisamochi A, Hino T, Ide T, Tanaka E, Koga Y. Genetic heterogeneity of the precore and the core promoter region of genotype C hepatitis B virus during lamivudine therapy. J Med Virol. 2004;72:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Maruyama T, Kuwata S, Koike K, Iino S, Yasuda K, Yotsuyanagi H, Moriya K, Maekawa H, Yamada H, Shibata Y. Precore wild-type DNA and immune complexes persist in chronic hepatitis B after seroconversion: no association between genome conversion and seroconversion. Hepatology. 1998;27:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Asahina Y, Izumi N, Uchihara M, Noguchi O, Nishimura Y, Inoue K, Ueda K, Tsuchiya K, Hamano K, Itakura J. Core promoter/pre-core mutations are associated with lamivudine-induced HBeAg loss in chronic hepatitis B with genotype C. J Hepatol. 2003;39:1063-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |