Published online Jun 14, 2005. doi: 10.3748/wjg.v11.i22.3339
Revised: June 9, 2004
Accepted: July 15, 2004
Published online: June 14, 2005
AIM: To investigate the cytotoxicity of the cytokine-induced killer (CIK) cells from the post-operation patients with primary hepatocellular carcinoma (HCC) to multidrug-resistant (MDR) cell of HCC both in vitro and in vivo.
METHODS: A drug-resistant cell line was established by culturing human HCC cell line Bel-7402 in complete RPMI 1640 medium with increasing concentrations of adriamycin from 10 to 2000 nmol/L. CIK cells were obtained by inducing the peripheral blood mononuclear cells with rhIFN-γ, monoclonal anti-CD3 antibody, rhIL-1α as well as rhIL-2, which were added into the culture. To detect the cytotoxicity of the CIK cells from HCC patients, the Bel-7402/R was taken as target (T) cells and CIK cells as effect (E) cells. Cytotoxic test was performed and measured by MTT. As to in vivo test, CIK cells were transfused into patients with HCC. The tumor specimens of the patients were obtained and immunohistochemistry was carried out to detect CD3, CD45, CD45RO as well as CD68.
RESULTS: A MDR 1 HCC cell line Bel-7402/R was established. Its MDR1 mRNA overexpressed which was shown by RT-PCR; the P-glycoprotein expression increased from 1.32% of parent cells to 54%. CIK cells expanded vigorously by more than 70-fold and the CD3+CD56+ increased by more than 600-fold after 3-wk incubation on average. The cytotoxicity of CIK from HCC patients to Bel-7402/R was about 50% and to L-02 below 10% (t = 8.87, P<0.01), the same as that of CIK from normal individuals. Each of the 17 patients received 1-5×1010 of CIK cell transfusion. No side effects were observed. After CIK treatment, the tumor tissue nodules formed and a large amount of lymphocytes infiltrated in the liver cancer tissue and CD3, CD45, CD45RO, and CD68 increased greatly which was shown by immunohistochemistry.
CONCLUSION: A stable MDR1 HCC cell line has been established which could recover from liquid nitrogen and CIK from HCC patients has strong cytotoxicity to MDR HCC cell. CIK adoptive immunotherapy is safe and has no side effects. Receivers improved their immunity to tumor evidently. CIK treatment may be a better choice for HCC patients after operation to prevent the recurrence, especially when tumors have developed drug resistance.
- Citation: Zhang YS, Yuan FJ, Jia GF, Zhang JF, Hu LY, Huang L, Wang J, Dai ZQ. CIK cells from patients with HCC possess strong cytotoxicity to multidrug-resistant cell line Bel-7402/R. World J Gastroenterol 2005; 11(22): 3339-3345
- URL: https://www.wjgnet.com/1007-9327/full/v11/i22/3339.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i22.3339
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors with a high mortality especially in China because of wide prevalence of HBV infection. Surgical operation is still the most effective and widely performed treatment, but the high frequency of recurrence after operation dramatically decrease the cure rate of HCC. To prevent recurrence of HCC, many measures have been taken such as regular chemotherapy after operation, intrahepatic arterial infusion of anticancer agents, transcatheter arterial embolization and recently, biotherapy[1-3] such as DC[4] cells and cytokine-induced killer (CIK) cells[5]. However, many patients’ prognosis is poor and the 5-year survival rate usually is below 15-30%. Besides other reasons, drug resistance of the tumors induced in the chemotherapy plays an important role, which in turn protects the tumors against the chemical agents[6]. Among the many known biological mechanisms of drug resistance, multidrug resistance (MDR) is of particular interest[7,8]. MDR is associated with amplification or overexpression of the MDR1 gene and the expression of a transmembrane glycoprotein of 170 ku termed P-glycoprotein (Pgp). As a drug pump, it excludes the chemical drugs out of tumor cells and causes treatment failure.
CIK cells are generated according to a novel protocol[9] and several authors[10-13] have demonstrated that CIK possesses versatile anti-tumor advantages including easier generation from T cells, stronger cytotoxicity to tumor as well as more enhanced in vivo proliferation potency while compared with other adoptive immunotherapies. Shi et al, and Wang et al, demonstrated that CIK treatment for HCC is effective and also safe. Now we show in this paper that multi-drug resistant HCC cell line Bel-7402/R, established by us, is also sensitive to CIK from HCC patients and positive pathological and immunohistochemical results were observed in one case whose tumor sample was verified as positive MDR1 expression by RT-PCR.
Seventeen HCC patients post-operation were enrolled in the study, including 16 cases of male and 1 female, aged 36-58 years with an average of 48.8 years. A written informed consent was obtained before CIK treatment which was certified by the Health Bureau of Hubei Province, China. Six of the patients were chosen randomly to take part in the in vitro test and six normal people from hospital staff were selected as normal controls.
All cell lines were grown[14] in complete medium (CM) namely RPMI 1640 (Gibco) with 100 mL/L calf serum, 25 mmol/L hepes, 2 mmol/L L-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin, and 50 μmol/L 2-mercaptoethanol (2-ME, all from Sigma). Some other agents were added according to research needs. Human HCC cell line Bel-7402 was a generous present from Professor Guanxin Shen, Department of Immunology, Tongji Medical School, Huazhong Science and Technology University. HCC cell line SMMC-7721 and embryo liver cell line L-02 were provided by the China Center for Type Culture Collection. Drug-resistance cell lines Bel-7402/R as well as SMMC-7721/R were induced by serially increasing adriamycin (ADM, Sigma) from 10 to 2000 nmol/L in the culture.
CIK cells were prepared as described according to Schmidt-Wolf[9]. Briefly, for in vivo treatment peripheral blood mononuclear cells (PBMCs) were collected by CS-3000 Plus blood cell separator (Baxter, USA) or for in vitro test prepared by ficoll separation from post-operation patients with HCC (HCC group) or from volunteers as control group (normal group). The cells were grown in CM and 1000 U/mL rhIFN-γ was added on d 0, after 24 h of incubation, 50 μg/L mouse monoclonal antibody (mAb) against CD3, 100 U/mL rhIL-1α (all from R&D Systems) and 1000 U/mL rhIL-2 (Sunshine Pharma. Co., Ltd, China) were added. Cell density was about 1×106/mL and fresh CM with IL-2 was replaced every 3 d. Cell phenotypes were identified by FCM on d 0, 7, 14, 25. Cytotoxicities to target were determined after d 14. Cells were transfused back to patients between d 14 and 21 based on the cell number.
Primers for MDR1 RT-PCR were designed based on the sequence in GenBank (NM000927), forward primer 5’-CAGGAGATAGGCTGGTTTGATGT-3’ and backward primer 5’-TTAGCTTCCAACCACGTGTAAATC-3’. PCR was performed according to molecular cloning protocol, briefly as follows: after the collection of cultured cells or mincing of HCC tissue into small pieces, RNA was extracted by TRIzol reagent. Extracted RNA was solved in diethyl pyrocarbonate-treated water (DEPC water). RT-PCR was performed in 50 μL of mixture containing: 1 μL of extracted RNA, 1 μL of 10 μmoL of both forward and backward primers (synthesized by Sangon, Shanghai, China), 5 μL of 10×buffer (100 mmol/L of Tris-HCl, pH 8.0, 500 nmol/L of KCl, 0.1% gelatin, 1% Triton X-100), 1 μL of 10 mmol/L dNTP, 4 μL of 25 mmol/L MgCl2, 2.5 U of ribonuclease inhibitor, 200 U of M-MLV RT enzyme, 2 U of Taq polymerase, and DEPC water was added to the final volume of 50 μL. The reaction mixture was kept at 42 °C for 30 min to synthesize first cDNA, and at 94 °C×3 min to denature DNA followed by 35 cycles of amplification consisting of 30 s of denaturing DNA at 94 °C, 30 s of annealing DNA at 58 °C and 30 s of extending DNA at 72 °C. β-actin served as control.
All samples were pretreated before analyzed by FCM analyzer (Coulter: EPICX XL). For cell phenotype identification, cells were incubated for 30 min with mAb against CD3, CD4, CD56, coupled to FITC or PE. For the detection of Pgp, cells were fixed with 4% paraformaldehyde for 10 min, incubated with mouse anti-human p-170 for 30 min at room temperature, and stained with goat antimouse IgG coupled to FITC. For Pgp function determination[15], cells were incubated with Rhodamine 123 (Sigma) for 1 h before being trypsinized and washed.
One hundred microliters of each parent or drug-resistant cells were distributed in 96-well culture microplates at 1×106/mL density in every well. ADM was added after a serial dilution from 40 μmoL to 36.25 nmoL. A triplicate of same samples was placed to control variation. Cells were incubated at 37 °C with 50 mL/L CO2 for 48 h prior to the addition of 10 μL of MTT[18,20] (5 g/L, Sigma). After another 4 h of incubation, the plate was centrifuged at 3000 r/min for 5 min, medium was discarded, 150 μL of DMSO was added to each well. Optical absorbance value was read at 570 nm by a microreader (BioRad 550, USA). Cell survival ratio (CSR) was calculated according to the formula: CSR = A570 experiment/A570 control×100%. The 50% inhibition concentration (IC50) was determined by concentration-CSR curve.
One hundred microliters of target cells were seeded in a microplate at a 1×105/mL density and 0.1 mL of effect cells at a 1×106/mL density were mixed into each well (to reduce the influence of effect cell number, we deliberately made the E:T = 10:1). After 4 h of incubation, 10 μL of MTT was added. And following treatments were the same as in drug -resistant test. Cytotoxicity rate (CR) was calculated by the following formula: CR = {1- (A570 experiment-A570 E control)/A570 T control}×100%.
Specimens were HE stained and observed under a microscope. Cell markers were demonstrated by immunohistochemistry according to S-P protocol on slides with mAb against CD3, CD45, CD45RO, CD68 (Neomarker) as first antibody, goat anti-mouse antibody as second antibody.
Analysis was performed by using Student’s t test, the data were expressed as mean±SD and P<0.05 was considered statistically significant.
The proliferation and phenotype of the PBMCs after CIK induction varied according to individual HCC patients. The cell number increase was more than 70-fold on average (n = 17) after 25-d incubation. CD3+CD56+ cells increased greatly by about 60-fold to more than 1400-fold with an average of more than 600-fold (Table 1).
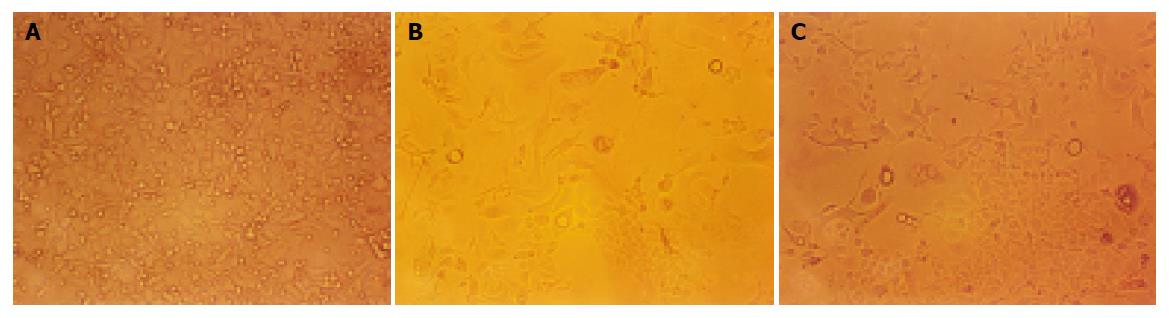
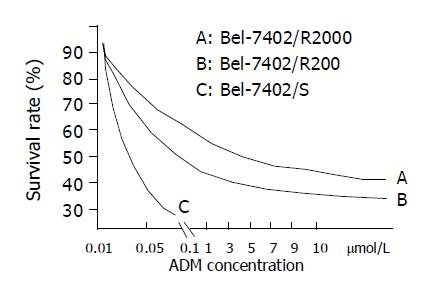
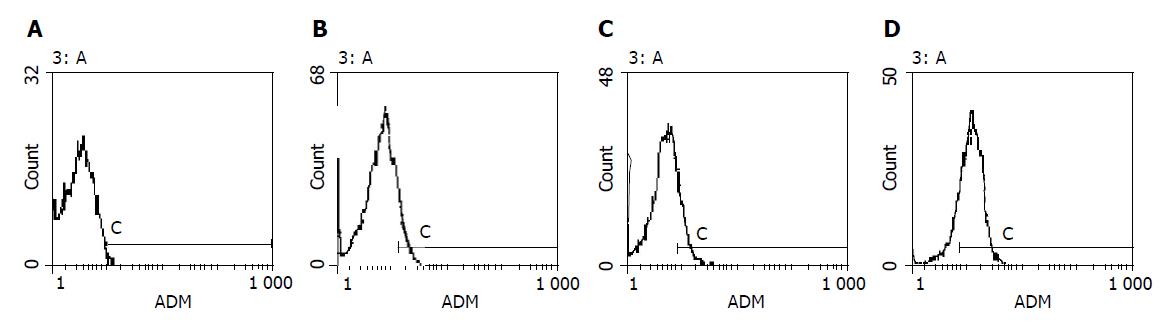
Bel-7402 HCC cell line under went drug-resistant induction test beginning with 10 nmol/L of ADM in the medium increased gradually to 200 or 2000 nmol/L. Cells showed a series of changes (Figure 1) such as enlarged, polynuclear, discontinuous in plasma membrane (Figure 1B). Most of them died then some “normal” cell islands (Figure 1C) appeared, the same as the parent cell (Figure 1A). We designated the induced cells which survived in 200 nmol/L of ADM as Bel-7402/R-200, and those survived in 2000 nmol/L of ADM, as Bel-7402/R-2000. After both grew in 2 mmol/L of ADM for 1 h, most of the former died and most of the latter still survived, indicating there might be some difference between the two. When MTT test was done to the parent cell and both drug-resistant cells, they showed different IC50 with ADM. IC50 of the parent Bel-7402 was 42 nmol/L, and of the induced cells, 2000 and 8000 nmol/L respectively (Figure 2). RT-PCR showed MDR1 overexpressed in induced cells and the parent cells generally gave negative results. Intracellular concentration of Rhodamine 123 was also determined by FCM (Figure 3), since Rhodamine 123 produces fluorescence itself and would be pumped out of cells if the Pgp existed. Bel-7402/R cells kept only 25.1%, 28.4% of Rhodamine 123 intracellularly each and the parent Bel-7402, 77.7%. Stained with anti-P-170 mAb (Figure 4), and determined by FCM, we found that only 1.32% of parent cells expressed P-170 and Bel-7402/R increased to 47%, 54% respectively.
Different from other reports, although it showed similar characters to Bel-7402/R in drug-resistance, the SMMC-7721/R only endured a smaller range of drug concentration(below 100 nmol/L of ADM) and it was difficult to recover from liquid nitrogen keeping.
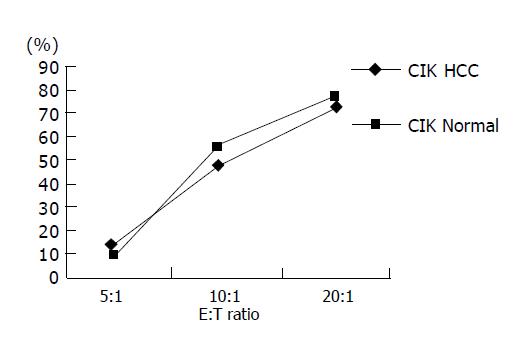
Shi et al, and Wang et al, have proven the CIK cells from HCC patients have stronger cytotoxicity to HCC cells and here we found that CIK both from normal persons and HCC patients had similar cytotoxicity to drug-resistant HCC cells (Figure 5) at different E:T ratios, so we chose E:T = 10:1 to avoid bias produced by effect cell number in the test. The experiment wells contained 104 target cells mixed with 105 effect cells. Effect control (E control) included 105 effect cells only and target control (T control), 104 target cells only. Cytotoxicity of CIK from HCC patients to Bel-7402/S was 33-68% with an average of 50.7%. While to Bel-7402/R it was 37-64% with an average of 52.3% (P>0.05), the same as normal group. Cytotoxicity CIK from HCC patients to L-02, an embryo liver cell line, was under 10%, indicating no side effect to normal liver cells (Tables 2 and 3).
| Patient Number | A value (rate of cytotoxicity) | |||
| 7402/sb(%) | 7402/Rd(%) | L-02bd(%) | E control | |
| 1 | 0.802 (33) | 0.743 (49) | 1.011 (12) | 0.485 |
| 2 | 0.913 (48) | 0.895 (47) | 1.201 (4) | 0.627 |
| 3 | 0.681 (46) | 0.599 (57) | 0.888 (14) | 0.379 |
| 4 | 0.630 (68) | 0.602 (60) | 0.959 (7) | 0.401 |
| 5 | 0.711 (58) | 0.802 (37) | 1.001 (12) | 0.479 |
| 6 | 0.987 (51) | 0.903 (64) | 1.302 (2) | 0.718 |
| T control | 0.554 | 0.508 | 0.595 | |
| 7402/s | 7402/R | |
| HCC | 50.7 | 52.3 |
| Normal | 57.1 | 54.7 |
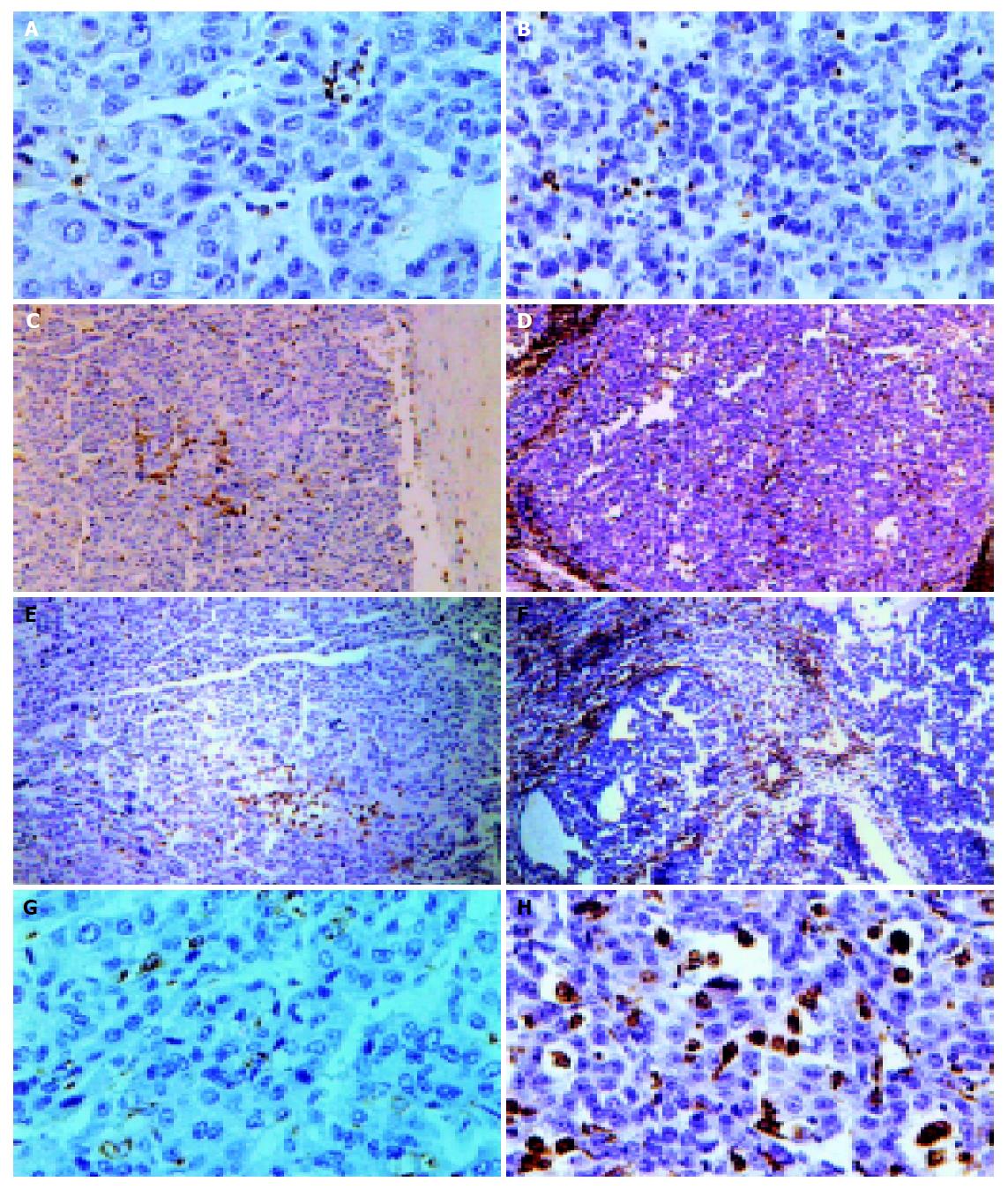
Seventeen cases of recurrent HCC patients post-operation were recruited into our study, most of them with relapsed bulk tumors and no effective treatment. The results showed that CIK treatment for HCC was safe, effective, without side effects(data reported elsewhere). Here we only focus on one case whose tumor had been demonstrated MDR1 overexpression by RT-PCR and immunohistochemistry assay before and after operation was carried out. The patient was a male, 56 years old, relapsed about one year after liver tumor resection with pneumal metastasis and cancer embolus in portal vein, and received CIK treatment. After transfused 1.3×1010 CIK cells, the patient had improvement of nausea, vomiting and the windy abdomen, as well as his appetite. His ascites abated too. After another 3 mo a second operation was performed and the tumor sample underwent pathological and immunohistochemical assay. Compared with the specimen before CIK treatment the tumor became nodular with a large amount of lymphocytes infiltration after treatment (Figure 6). CD3, CD45, CD45RO, CD68 positive cells increased vigorously (Table 4 and Figure 6) to 41, 283, 44, 77/HPF each from 0.1, 15, 3, 8/HPF before CIK treatment indicating the anti-tumor immunity was enhanced significantly since CD3, CD45 represent T cells, CD45RO is the marker of T memory subset, while CD68 is the marker of mono/macrophage.
| CD45 | CD3 | CD45RO | CD68 | |
| 1 | 15.4 cells/HPF | 0.15 cells/HPF | 2.9 cells/HPF | 8.0 cells/HPF |
| 2 | 283 cells/HPF | 41.3 cells/HPF | 44.1 cells/HPF | 77.7 cells/HPF |
| x-fold | 18.4 | 275 | 15.2 | 9.7 |
Other than transgene models, several drug-resistant HCC cell lines induced by ADM have been established[17-19]. In our laboratory, we found that Bel-7402 is easier and has a wider range in drug-dose to induce drug-resistance than smmc-7721, as the latter is difficult to grow in >100 nmol/L of ADM and to recovered from liquid nitrogen preservation. Bel-7402/R still keep its resistance even revived from the liquid nitrogen and so it is easy to use. Our research demonstrated that MDR1 gene expression is one of important mechanisms of drug-resistance in our Bel-7402/R. At the same time, that Bel-7402/R-2000 was more resistant to the drug than Bel-7402/R200 reminded us that there should be some other reasons to be disclosed.
A number of studies[20,21] have illuminated that CIK possessed much stronger cytotoxicity than LAK to several kinds of cancer such as leukemia, renal cell carcinoma, melanoma[22], and proved that dendritic cells increased in serum of host after transfusion of CIK to HCC patients. Here we demonstrated CIK cells from HCC patients could equally kill drug-resistant HCC cell line as those from normal. One case of in vivo study also gave same results. Immunohistochemistry of specimens revealed large amounts of lymphocytes infiltrating into tumor; CD3+, CD45RO+, CD68+ cells were recruited there in great numbers. These observations may imply activation of anti-tumor immunity since CD45RO+ represents T memory subset while CD68+ is the marker of monocyte/macrophage[23]. Anti-CD3Ab can activate CD45RO+ T subset[24], so we deduced that the increase of CD45RO+ cells in the tumor locals may come from CIK.
CIK cell killing drug-resistant HCC cell line is of much benefit to HCC treatment. HCC is easy to induce drug-resistance. Because of tendency of metastasis and recurrence of HCC, post-operation chemotherapy is necessary. Since p-170 pumps drugs out of the cells, chemotherapy is not only useless to the drug-resistant tumor but produces the side effects to the host as well. CIK would be the better choice to kill the drug-resistant tumor. We suggest to perform CIK treatment to eradicate the remaining tumor cells after bulk tumor loads are removed by operation.
It is interesting that while CIK kills the tumor cells vigorously it has little effect on L-02 cells. Similar results were reported by Schmidt-Wolf et al[25]. They found that despite high cytotoxic activity of CIK against lymphoma cells, they had little toxicity against a subset of normal human hematopoietic progenitor cells. Some difference between Bel-7402 and L-02 has been reported recently by Shao et al[26], that LAPTM4B, a newly found HCC related antigen, was expressed in Bel-7402 but not in L-02. A polyclonal antibody against LAPTM4b has been produced. Monoclonal antibody against it is in process of production. It may be helpful to solve the problem.
Co-first-authors: You-Shun Zhang and Fang-Jun Yuan
| 1. | Zhang YS, Dai ZQ. Advances in Hepatoma Biotherapy Research. Zhongguo Xiandai Putong Waike Jinzhan. 2001;4:193-198. |
| 2. | Kline NE. Promoting patient safety through the development of a pediatric chemotherapy and biotherapy provider program. J Pediatr Oncol Nurs. 2004;21:65-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Tan GH, Wei YQ, Tian L, Zhao X, Yang L, Li J, He QM, Wu Y, Wen YJ, Yi T. Active immunotherapy of tumors with a recombinant xenogeneic endoglin as a model antigen. Eur J Immunol. 2004;34:2012-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Rice AM, Jones KL, Hart DN. DC preparations for therapy. Cytotherapy. 2004;6:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Hongeng S, Petvises S, Worapongpaiboon S, Rerkamnuaychoke B, Pakakasama S, Jootar S. Generation of CD3+ CD56+ cytokine-induced killer cells and their in vitro cytotoxicity against pediatric cancer cells. Int J Hematol. 2003;77:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Bonin S, Pascolo L, Crocé LS, Stanta G, Tiribelli C. Gene expression of ABC proteins in hepatocellular carcinoma, perineoplastic tissue, and liver diseases. Mol Med. 2002;8:318-325. [PubMed] |
| 7. | van Brussel JP, van Steenbrugge GJ, Romijn JC, Schröder FH, Mickisch GH. Chemosensitivity of prostate cancer cell lines and expression of multidrug resistance-related proteins. Eur J Cancer. 1999;35:664-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Warmann S, Göhring G, Teichmann B, Geerlings H, Pietsch T, Fuchs J. P-glycoprotein modulation improves in vitro chemosensitivity in malignant pediatric liver tumors. Anticancer Res. 2003;23:4607-4611. [PubMed] |
| 9. | Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174:139-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 476] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Schmidt-Wolf GD, Negrin RS, Schmidt-Wolf IG. Activated T cells and cytokine-induced CD3+CD56+ killer cells. Ann Hematol. 1997;74:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Schmidt-Wolf IG, Lefterova P, Mehta BA, Fernandez LP, Huhn D, Blume KG, Weissman IL, Negrin RS. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol. 1993;21:1673-1679. [PubMed] |
| 12. | Kaneko T, Fusauch Y, Kakui Y, Okumura K, Mizoguchi H, Oshimi K. Cytotoxicity of cytokine-induced killer cells coated with bispecific antibody against acute myeloid leukemia cells. Leuk Lymphoma. 1994;14:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Mehta BA, Schmidt-Wolf IG, Weissman IL, Negrin RS. Two pathways of exocytosis of cytoplasmic granule contents and target cell killing by cytokine-induced CD3+ CD56+ killer cells. Blood. 1995;86:3493-3499. [PubMed] |
| 14. | Zhang YS, Huang L, Yuan FJ, Wang B, Dai ZQ. Research on effects of tumor vaccine to mouse hepatocellular carcinoma. Yunyang Yixueyuan Xuebao. 2002;21:8-12. |
| 15. | Shapiro AB, Ling V. The mechanism of ATP-dependent multidrug transport by P-glycoprotein. Acta Physiol Scand Suppl. 1998;643:227-234. [PubMed] |
| 16. | Zhang YS, Yao L, Huang L, Wang B, Dai ZQ. Observation on cytotoxicity of CD3AK. Yunyang Yixueyuan Xuebao. 1999;18:200-201. |
| 17. | Nakajima A, Yamamoto Y, Taura K, Hata K, Fukumoto M, Uchinami H, Yonezawa K, Yamaoka Y. Beneficial effect of cepharanthine on overcoming drug-resistance of hepatocellular carcinoma. Int J Oncol. 2004;24:635-645. [PubMed] |
| 18. | He L, Liu GQ. Effects of various principles from Chinese herbal medicine on rhodamine123 accumulation in brain capillary endothelial cells. Acta Pharmacol Sin. 2002;23:591-596. [PubMed] |
| 19. | Chu TM, Lin TH, Kawinski E. Detection of soluble P-glycoprotein in culture media and extracellular fluids. Biochem Biophys Res Commun. 1994;203:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Schmidt-Wolf IG, Finke S, Trojaneck B, Denkena A, Lefterova P, Schwella N, Heuft HG, Prange G, Korte M, Takeya M. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin-2 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br J Cancer. 1999;81:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Alvarnas JC, Linn YC, Hope EG, Negrin RS. Expansion of cytotoxic CD3+ CD56+ cells from peripheral blood progenitor cells of patients undergoing autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2001;7:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Märten A, Ziske C, Schöttker B, Renoth S, Weineck S, Buttgereit P, Schakowski F, von Rücker A, Sauerbruch T, Schmidt-Wolf IG. Interactions between dendritic cells and cytokine-induced killer cells lead to an activation of both populations. J Immunother. 2001;24:502-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993;81:1607-1613. [PubMed] |
| 24. | Gold JE, Zachary DT, Osband ME. Adoptive transfer of ex vivo-activated memory T-cell subsets with cyclophosphamide provides effective tumor-specific chemoimmunotherapy of advanced metastatic murine melanoma and carcinoma. Int J Cancer. 1995;61:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Schmidt-Wolf IG, Lefterova P, Johnston V, Scheffold C, Csipai M, Mehta BA, Tsuruo T, Huhn D, Negrin RS. Sensitivity of multidrug-resistant tumor cell lines to immunologic effector cells. Cell Immunol. 1996;169:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Shao GZ, Zhou RL, Zhang QY, Zhang Y, Liu JJ, Rui JA, Wei X, Ye DX. Molecular cloning and characterization of LAPTM4B, a novel gene upregulated in hepatocellular carcinoma. Oncogene. 2003;22:5060-5069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 146] [Article Influence: 6.6] [Reference Citation Analysis (0)] |