Published online May 14, 2005. doi: 10.3748/wjg.v11.i18.2838
Revised: November 18, 2004
Accepted: January 5, 2005
Published online: May 14, 2005
AIM: To study the method of dissociation, culture and investigate its morphologic changes in vitro of interstitial cells of Cajal (ICC).
METHODS: Enzymatic digestion and Ficoll density centrifu-gation were used to dissociate ICC from the ileal segment of mice. Factors including contamination, Ca2+, Mg2+ and collagenase, and stem cell factor, etc., were investigated. ACK2, the antibody of c-kit, was used to identify the cultured ICC. Both light microscope and fluorescence microscope were used to observe the changes of ICC in vitro.
RESULTS: The method for dissociation and culture of ICC in vitro was successfully established. After 24 h, cultured ICC exhibited a few axis-cylinders, and longer axis-cylinders were observed to form synapse of each other after 3 d. More widespread connections formed within 7 d in vitro. The changes of its morphologic character were obvious within 7 d; however, there were no obvious morphologic changes after 30 d.
CONCLUSION: Many factors can influence the dissociation and culture of ICC.
-
Citation: Li CX, Liu BH, Tong WD, Zhang LY, Jiang YP. Dissociation, culture and morphologic changes of interstitial cells of Cajal
in vitro . World J Gastroenterol 2005; 11(18): 2838-2840 - URL: https://www.wjgnet.com/1007-9327/full/v11/i18/2838.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i18.2838
Interstitial cells of Cajal (ICC) are the pacemakers in gastrointestinal (GI) muscles, and also transduce inputs from the enteric nervous system. In the intestine, slow wave generation has been linked to the presence of a layer of ICC within the Auerbach’s plexus region[1-3]. ICC are mesenchymal cells that have precursor cells in common with smooth muscle cells, and possess the Kit tyrosine kinase membrane receptor as their special characteristics[4-6]. Although there are many researches on ICC now[5-7], the procedure in isolation and culture of ICC still has many difficulties that should be studied and discussed. Their morphologic changes during short-term culture in vitro have no clear explanation by now. In this study, the method of dissociation and culture of ICC were established and discussed. The process of their morphologic changes in vitro was observed at least for 30 d. The method of ICC culture should help us make deeper research of ICC in future.
The BALB/C mice (15-20 d old) of either sex were from the Experimental Animal Center of Daping Hospital, Chongqing. Animals were not given food for 24 h prior to the experiment. Mice were killed by cervical dislocation. The ileal segment about 10 cm proximal to the ileocecal junction was removed. The muscularis propria was gently peeled from the mucosa and placed in Ca-free Hank’s balanced salt solution (Hyclone) with 1% antibiotic-antimycotic (Sigma). Subsequently, the segment was washed thrice through the duct with Ca2+-free Hank’s balanced solution (1% antibiotic-antimycotic). The segment was opened flat by cutting along the mesenteric line and pinned flat with the mucosa facing the dissecting wood board. The dissected muscle was carefully cut into small pieces (1-2 mm3) for enzymatic digestion.
The muscle pieces were incubated at 37 °C in collagenase-based dissociation solution containing 1.3 mg/mL collagenase (type II, Sigma), 2 mg/mL bovine serum albumin (BSA, Sigma), 2 mg/mL trypsin inhibitor (Sigma), and 0.27 mg/mL ATP (Sigma), 10 mL of calcium-containing Hank’s balanced salt solution (Hyclone). The pH was adjusted to 7.0 with 0.1 mol/L NaOH. After 30 min at 37 °C without shaking the water bath, the tissue was bluntly triturated with pipette every 3 min until single cells were obtained for approximately 10 min. After passing through the sieve (size: #200), all cell suspension was layered on the surface of a 200 g/L Ficoll density cushion and spun at 15 r/min for 15 min. The cell band located at the interface was transferred to a new container and resuspended with M199 medium with 10% fetal bovine serum (Hyclone), 1% antibiotic to the desired density (about 2×106). The suspension was plated into Falcon petri dishes (with collagen-coated coverslips) on the bottoms. The cells were maintained in 50 mL/L CO2 at 37 °C.
ICC were observed under Olympus inverted microscope, with 100×, 200× or 400× power. The images were captured with Olympus color video camera and then directly recorded in the computer.
Cultured cells were prepared for immunofluorescence labeling by fixation in acetone (4 °C, 10 min). After fixation, the cells were incubated in normal goat serum for 1 h (10% in PBS), and then at 4 °C with a rat monoclonal antibody specific for Kit protein (ACK2, 5 mg/mL) in PBS overnight. Immunoreactivity was detected using fluorescein isothiocyanate (FITC)-conjugated secondary antibody (FITC-anti-rat, Zhongshan, China; diluted 1:100 in PBS, 1 h, room temperature). Control cultures were prepared in a similar manner, but ACK2 was omitted from the incubation solution. The cells were examined with a LEICA TCS SP2 (Germany) confocal microscope with an excitation wavelength appropriate for FITC (488 nm). All images were captured and recorded in the computer.
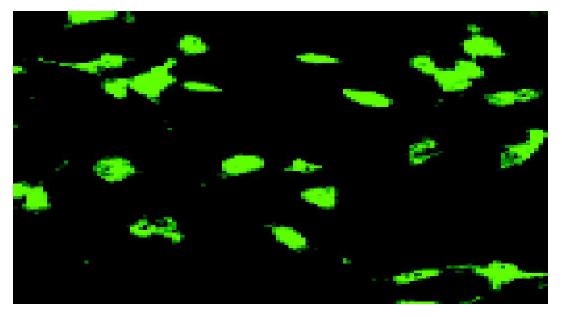
We performed immunofluorescence analysis to determine the morphology of cells that displayed Kit immunoreactivity. The cells showed fusiform cell bodies, prominent nuclei, and multiple thin processes extending from the nuclear region on light micrograph. ICC, with this morphology expressed Kit-like immunoreactivity, could form network of each other as shown on the fluorescence micrograph (Figure 1).
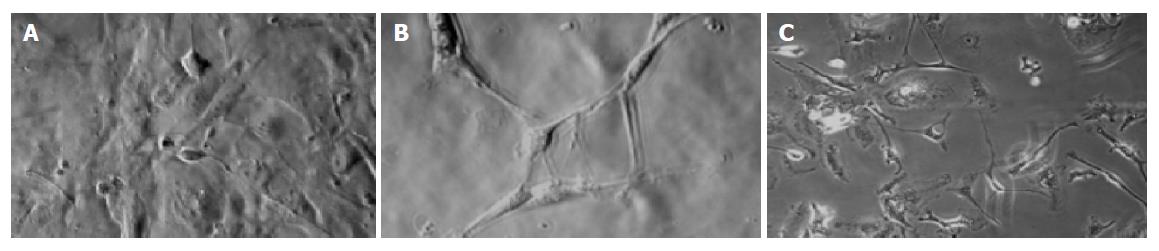
After 24 h in culture, most cells showed fusiform cell bodies, large and prominent nuclei, and multiple, short and thin processes extending from the nuclear region (Figure 2A). Cells with this morphology were easily distinguished from the smooth muscle cells.
After 72 h in culture, cells with more and longer processes extending from the nuclear region could be observed (Figure 2B). Some cells began to form a characteristic network of each other.
After 7 d in culture, cells with more multiple and longer processes could be observed under phase-contrast microscope (Figure 2C). More characteristic networks could be found and the morphologic changes could be observed even after 30 d.
In GI tract, ICC are intimately connected with smooth muscle cells, fibrocytes and nerve fasciculi. Some methods can isolate and culture ICC[5-7]. Many difficulties should be overcome in the procedure of culturing ICC. However, no detailed research on the method of ICC culture is available before this study. In this study, we found that following factors should be discussed in the procedure of ICC culture.
If the culture medium is contaminated, few cells can be adhered to the coverslips at first, and no cells could be obtained after 3 d. The sources of infection mainly include E. coli, staphylococci, eumycetes, etc. In the study, no food was given for at least 12-24 h prior to the experiment. It is helpful to make the small intestine clean. In the procedure of isolating ICC, sufficient washing of the small intestine should be equally important to reduce contamination. Moreover, antibiotics (1% antibiotic-antimycotic was used in the study) should be added in the washing solution and culture medium. At last, strict aseptic manipulation should be mentioned in the process of cell culture.
Solution containing no Ca2+ solution is very important to decrease the connection of ICC to other cells and matrix. For good dissociation of tissue, it is unavoidable to expose ICC to solution containing no Ca2+[5-8]. However, when it is re-exposed to normal Ca2+ levels, overshoot of cellular Ca2+ levels would occur. We suggest that tissues exposed to low Ca2+ levels before placement in medium M199 (about 2.5 mmol/L Ca2+) should be helpful to reduce the overshoot of Ca2+. To get the desired quantity of ICC, shortening the time in vitro and minimizing exposure to solution containing no Ca2+ should be mentioned.
Enzyme digestion is necessary in releasing ICC from tissues. Collagenase was used to isolate ICC in this study, while some other enzymes have been used either alone or in combination in other researches[5]. BSA and inhibitor of trypsin can be added in the solution to retain collagenase activity. We found that collagenase worked better in the presence of Ca2+. Besides the component of enzyme solution, appropriate time of the enzyme digestion should also be mentioned. We found that insufficient digestion would get few single cells, while sufficient digestion would not get desired quantity of ICC as well. In this study, 30-min digestion at 37 °C was conducted to isolate ICC.
In the study, the culture medium was changed after 24 h in culture. Then the medium was changed every 3-4 d. Twenty-four hours would be enough for ICC to adhere to the coverslips. Changing the culture medium in time can provide nutrient for ICC.
Stem cell factor (SCF) is the ligand of the c-kit (CD117). The signal stimulated by c-kit/SCF is essential to maintain the phenotype of ICC[8-10]. In the culture medium M199, murine recombinant SCF was added at the concentration of 5 ng/mL in our study. Both the results in our study and other works showed that SCF is essential to obtain and maintain ICC in good condition[8,11-13]. In the study, we found that the c-kit with SCF was expressed on ICC in a dose-dependent manner.
In the study, the isolated ICC could contact each other. Multiple networks of ICC could also be found after about 7 d in culture, suggesting that the specific network of ICC is essential to maintain and realize its phenotype function.
It has been confirmed that c-kit only emerges on ICC and mast cells (-5%) in GI[10]. We consider that almost all c-kit-stained cells should be ICC. In the study, ICC were identi-fied by immunofluorescence with c-kit-specific antibody ACK2.
After ICC were plated in M199 medium, the cells became round and could be adhered to the coverslips within 24 h in culture. The cell body was generally triangular or stellar and with a large nucleus surrounded by little cytoplasm. In the first 3 d, there were generally 3-5 primary processes, which could branch into secondary and tertiary processes. In the study, the isolated ICC could contact each other and form multiple networks within 7 d. These specific networks of ICC are the same as in vivo, suggesting that it is essential to maintain and realize its phenotype and function.
The detailed method of ICC culture can contribute to its deeper research.
| 1. | Ward SM, Ordög T, Bayguinov JR, Horowitz B, Epperson A, Shen L, Westphal H, Sanders KM. Development of interstitial cells of Cajal and pacemaking in mice lacking enteric nerves. Gastroenterology. 1999;117:584-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Hanani M, Freund HR. Interstitial cells of Cajal--their role in pacing and signal transmission in the digestive system. Acta Physiol Scand. 2000;170:177-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Takaki M. Gut pacemaker cells: the interstitial cells of Cajal (ICC). J Smooth Muscle Res. 2003;39:137-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 131] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Lee JC, Thuneberg L, Berezin I, Huizinga JD. Generation of slow waves in membrane potential is an intrinsic property of interstitial cells of Cajal. Am J Physiol. 1999;277:G409-G423. [PubMed] |
| 5. | Rich A, Miller SM, Gibbons SJ, Malysz J, Szurszewski JH, Farrugia G. Local presentation of Steel factor increases expression of c-kit immunoreactive interstitial cells of Cajal in culture. Am J Physiol Gastrointest Liver Physiol. 2003;284:G313-G320. [PubMed] |
| 6. | Huizinga JD, Robinson TL, Thomsen L. The search for the origin of rhythmicity in intestinal contraction; from tissue to single cells. Neurogastroenterol Motil. 2000;12:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Ordög T, Redelman D, Miller LJ, Horváth VJ, Zhong Q, Almeida-Porada G, Zanjani ED, Horowitz B, Sanders KM. Purification of interstitial cells of Cajal by fluorescence-activated cell sorting. Am J Physiol Cell Physiol. 2004;286:C448-C456. [PubMed] |
| 8. | Wu JJ, Rothman TP, Gershon MD. Development of the interstitial cell of Cajal: origin, kit dependence and neuronal and nonneuronal sources of kit ligand. J Neurosci Res. 2000;59:384-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Gibbons SJ, Rich A, Distad MA, Miller SM, Schmalz PF, Szurszewski JH, Sha L, Blume-Jensen P, Farrugia G. Kit/stem cell factor receptor-induced phosphatidylinositol 3'-kinase signalling is not required for normal development and function of interstitial cells of Cajal in mouse gastrointestinal tract. Neurogastroenterol Motil. 2003;15:643-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Epperson A, Hatton WJ, Callaghan B, Doherty P, Walker RL, Sanders KM, Ward SM, Horowitz B. Molecular markers expressed in cultured and freshly isolated interstitial cells of Cajal. Am J Physiol Cell Physiol. 2000;279:C529-C539. [PubMed] |
| 11. | Kim YC, Koh SD, Sanders KM. Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine. J Physiol. 2002;541:797-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Ward SM, Sanders KM, Hirst GD. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol Motil. 2004;16 Suppl 1:112-117. [PubMed] |
| 13. | Wedel T, Spiegler J, Soellner S, Roblick UJ, Schiedeck TH, Bruch HP, Krammer HJ. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology. 2002;123:1459-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 235] [Article Influence: 10.2] [Reference Citation Analysis (0)] |