Published online May 14, 2005. doi: 10.3748/wjg.v11.i18.2822
Revised: May 28, 2004
Accepted: June 24, 2004
Published online: May 14, 2005
AIM: To study the effect of sulindac on colon cancer induction in mice.
METHODS: The chemo-preventive action of 80 ppm sulindac fed during initiation and post-initiation and 100 ppm sulindac fed during progressive stages of induction of colon carcinogenesis in mice was investigated using 1,2-dimethylhydrazine (DMH). Using the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) technique and PCNA immunohistochemical staining, we observed the apoptotic and proliferative cell density changes at different carcinogenic stages and the effect of sulindac on these two phenomena.
RESULTS: Dietary sulindac significantly inhibited the incidence of colonic neoplasmas in mice. Compared with the control group, feeding sulindac during initiation and post-initiation stages inhibited the incidence by 46.7-50.4%, and feeding sulindac during progressive stages inhibited the incidence by 41.1%. Animals that were fed sulindac showed less serious pathological changes than those that were fed the control diet (P<0.01, H = 33.35). There was no difference in the density of proliferating cells among those groups which were or were not fed sulindac. In the same period, feeding sulindac resulted in a higher density of apoptotic cells than feeding control diet.
CONCLUSION: Sulindac has an anti-carcinogenic function in mice. Its effect on preventing colon carcinogenesis is better than its effect on treating established tumors. By inducing apoptosis, sulindac inhibited the development of colon cancer and delayed canceration. Sulindac has no effect on proliferation. The anti-carcinogenic properties of sulindac are most effective in the moderate and severe stages of dysplasia and canceration.
- Citation: Sun BC, Zhao XL, Zhang SW, Liu YX, Wang L, Wang X. Sulindac induces apoptosis and protects against colon carcinoma in mice. World J Gastroenterol 2005; 11(18): 2822-2826
- URL: https://www.wjgnet.com/1007-9327/full/v11/i18/2822.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i18.2822
Large intestinal carcinoma is a common malignant tumor of the digestive system and its incidence is in an ascending trend. More and more evidence shows that dietary factors play an important role in the progression of large intestinal carcinoma. Sulindac, a type of non-steroidal anti-inflammatory drug (NSAID), gets metabolized into a sulindac sulfone[1]. In recent years, more and more studies concerning sulindac in preventing familial adenomatous polyposis and influencing large intestinal cancer cellular proliferation, differentiation and apoptosis have been published[2]. However, the anti-tumor mechanism of the effects of sulindac during different stages of large intestinal canceration remains to be elucidated. In our study, 1,2-dimethylhydrazine (DMH) was used as a model agent experimentally to induce large intestinal carcinoma in mice in order to observe the dynamic effects of sulindac’s influence on the apoptotic and proliferative cell density; whether the anti-tumor effects of sulindac are due to induction of the tumor cell apoptosis or not was examined. Furthermore, prevention by sulindac in the early stage of tumor formation and its curative effect in an advanced stage will be assessed. In summary, experimental data relating to the ability of sulindac to prevent and cure large intestinal cancer will be offered in this study.
One hundred and sixty KUNMING strain mice, male, 5 wk of age, 24-30 g, were randomly divided into the following groups: model-agent (48), sulindac preventative (48), sulindac curative (40) and control (24) and used in our study after feeding 1 wk.
Normal diet (AIN-76A) and sulindac diet were produced by the Animal Department of Tianjin Chinese Traditional Medical University. The animals were given food containing sulindac at 80 mg/kg in the sulindac-preventative group and at 100 mg/kg in sulindac-curative group. All food was prepared once every 3-4 wk and maintained at room temperature.
Reagents used in this study were DNH and sulindac (Sigma Inc., USA), TUNEL assay (BM Inc., Germany) and ABC immunohistchemical compounds (Vector Inc., USA).
Model-agent group: DMH dissolved in normal saline was injected subcutaneously into the model-agent group mice at a dosage of 20 mg/kg. The mice received the normal diet and were killed at the 12th (10), 18th (10), 24th (10) and 32nd (18) wk after injection.
Sulindac preventative group: the mice were fed a Sulindac diet with the dosage of 80 mg of sulindac/kg for 26 wk at the time of DNH injection and were killed at the 12th, 18th, 24th and 32nd wk.
Sulindac curative group: the mice were fed a Sulindac diet with the dosage of 100 mg of sulindac/kg starting at 8 wk after the DNH injection and for 24 more wk. The mice were killed at the end of the 32nd wk.
Control group: the mice were fed normal diet and the same quantity of normal saline was injected. The mice were killed at the end of the 18th and 32nd wk.
The mice were killed by cervical dislocation and the whole large intestine and important organs were removed and examined visually. The tissues were fixed in 40 g/L formaldehyde, dehydrated, paraffin-embedded and sectioned at 5 μm thickness. The investigation included three parameters.
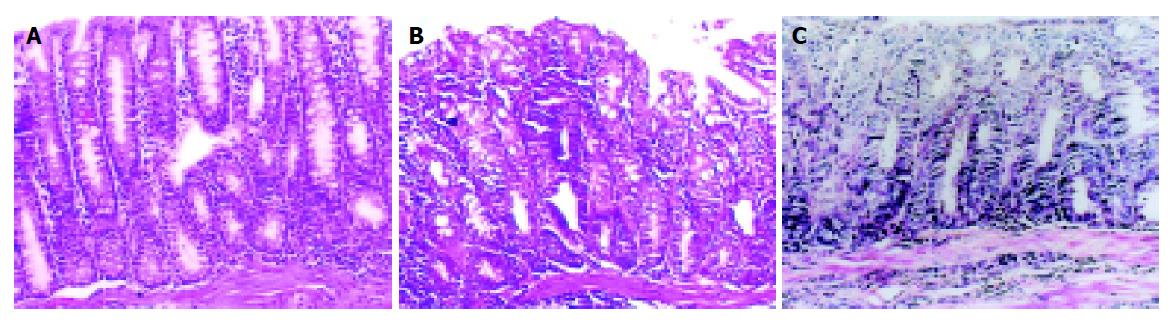
Pathological observation The large intestine was opened lengthways and the number, size and infiltrative depth of the tumors were observed. The grade of the tissue lesions under microscopic examination included mild, moderate and severe dysplasia and cancer (Figures 1A-1C).
Distribution and density of apoptosis The distribution and density of apoptosis were demonstrated with TUNEL.
Distribution and density of proliferation The distribution and density of proliferation were recorded by counting cells in a G1/S phase determined by PCNA immunohistochemistry.
TUNEL staining The sections were deparaffinized and put into 3% hydrogen peroxide for 20 min at room temperature. After nonspecific binding sites were blocked, the sections were incubated for 60 min at 37 °C with TUNEL and stained with 3,3’-diaminobenzidine(DAB) chromogen for 10 min at room temperature. Finally, all of the sections were counterstained with hematoxylin. The sections were rinsed with PBS after every step.
PCNA immunohistochemical staining Five-micrometer formalin-fixed paraffin- embedded tissue sections were mounted on poly-L-lysine slides. The slides were air-dried and the tissue deparaffinized. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in 50% methanol for 10 min at room temperature. The sections were rehydrated and washed with PBS and then pretreated with citrate buffer (0.01 mol/L citric acid, pH 6.0) for 20 min at 100 °C in a microwave oven. Then the sections were incubated overnight at 4 °C with mouse monoclonal anti-PCNA. The sections then were rinsed with PBS, incubated with biotinylated goat anti-mouse IgG for 20 min at 37 °C, incubated with the third antibody and then stained with DAB. Finally, all of the sections were counterstained with hematoxylin.
Method of counting The number of positive cells in the sections with TUNEL and PCNA were counted under microscope magnified 400× with a 16D gridding. All positive cells in the 16D gridding were counted except those cells that lay to the right and on the line at the bottom. More than five 16D-gridding microscopic fields were counted in 1 section. The average was considered as the positive-cell density. The area of a 16D-gridding is about 0.1 mm2. The density of proliferation and apoptosis was calculated according to the positive cell number in 1 mm2.
Rank sum test and analysis of variance were performed using SPSS statistical analytic software. P<0.05 was considered significant.
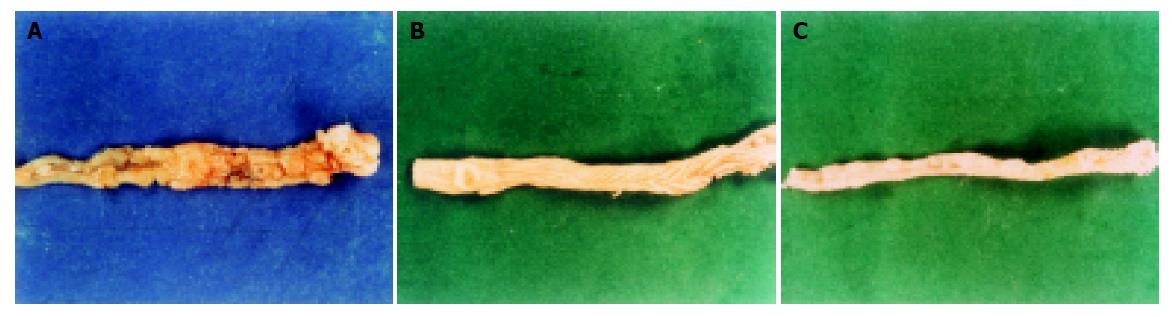
Macroscopic examination Single or multiple nodes whose diameters ranged from 0.1 to 0.3 cm situated on the surface of the large intestinal mucosa were found in 22.2% of the model-agent group at the 24th wk. Multi nodes with the maximum diameter of 1.8 cm were demonstrated in 86.7% of the mice at the 32nd wk with necrosis and ulcer formation in some nodes. The nodes usually were seen at the distal end of the large intestine. Thickening of the large intestinal wall, rigidity, and adhesion were observed in some mice. The large intestinal mucosa of the mice showed mild, moderate and severe dysplasia in turn in model-agent group, preventive group and curative group during the progression of carcinogenesis and large intestinal carcinomas developed in some mice (Figures 2A-2C). One mouse in the model-agent group died of lung infection and three mice of the sulindac curative group died of intestinal obstruction from a tumor. Tables 1 and 2 show the number and size of the tumors at the 24th and 32nd wk.
| Wk | n | ≤5 mm | ≤10 mm | ≤15 mm | >15 mm | Incidence |
| 24th | 9 | 1 | 1 | 0 | 0 | 22.2 |
| 32nd | 15 | 2 | 6 | 3 | 2 | 86.7 |
| Control | 12 | 0 | 0 | 0 | 0 | 0 |
| Wk | n | 0 | ≤1 | ≤2 | ≤3 | ≤4 | ≤5 | >5 |
| 24th | 9 | 7 | 0 | 1 | 1 | 0 | 0 | 0 |
| 32nd | 15 | 2 | 0 | 5 | 2 | 1 | 3 | 2 |
Microscopic examination Mild, moderate and severe dysplasia were observed in the large intestinal mucosa of the mice during the process of tumor induction. Cancer development in the large intestine appeared by the 24th wk. The histological type of these tumors was mainly adenocarcinoma with only few adeno-squamous cell carcinomas. The infiltrative depth of tumors reached the muscle and outer layer. Three mice had liver metastatic foci. The pathological changes in the large intestinal mucosa at various times are shown in Table 3. The incidence of large intestinal carcinoma was found in 66.7% of the model-agent group and 20% in the sulindac preventative group at the 24th wk, at 32nd wk in 93.3% in the model-agent group, 42.9% in the sulindac preventative group and 52.2% in the sulindac curative group.
| Week | n | Normal | Dysplasia | Adenocarcinoma | |||
| Mild | Moderate | Severe | Intramucouscarcinoma | Infiltrative carcinoma | |||
| 12th | 10 | 2 | 3 | 4 | 1 | 0 | 0 |
| 18th | 10 | 0 | 2 | 4 | 4 | 0 | 0 |
| 24th | 9 | 0 | 0 | 1 | 2 | 5 | 1 |
| 32nd | 15 | 0 | 0 | 0 | 1 | 2 | 12 |
Apoptotic and proliferative cell density developed an increasing trend as the degree of dysplasia increased. In the cancerous stage, the density of apoptosis was lower than it was in a dysplasia stage. On the contrary, the proliferative cells density increased and the ratio between apoptosis and proliferation decreased.
The density of apoptosis in the sulindac-preventative group was higher than in the other groups and there was a statistically significant difference during moderate dysplasia and the cancerous stage. The density of apoptosis in the sulindac-curative group was also higher than it was in the other groups, but there was only a statistically significant difference during the cancerous stage. The density of apoptosis in the sulindac-preventative group was higher than that in the sulindac-curative group and there was a significant difference during the moderate dysplasia and canceration stages.
The difference in the density of proliferation between sulindac-preventative group and sulindac-curative group was not statistically significant during the whole cancerogenic stage but the proliferative cell density in the sulindac-preventive group and sulindac-curative group was higher than in the control group. The proliferative cell density in the model-agent group was higher than in sulindac- preventative group, sulindac-curative group and control groups (Tables 4, 5, Tables 6, and 7).
| Group | n | Proliferative cell density (mean±SD) | Apoptotic cell density (mean±SD) | Ratio (apoptosis/proliferation) |
| Model | 8 | 159.0±45.9 | 61.0±24.6 | 0.38 |
| Preventive | 3 | 155.8±31.9 | 88.5±14.6 | 0.57 |
| Curative | 5 | 159.8±22.7 | 64.2±19.1 | 0.4 |
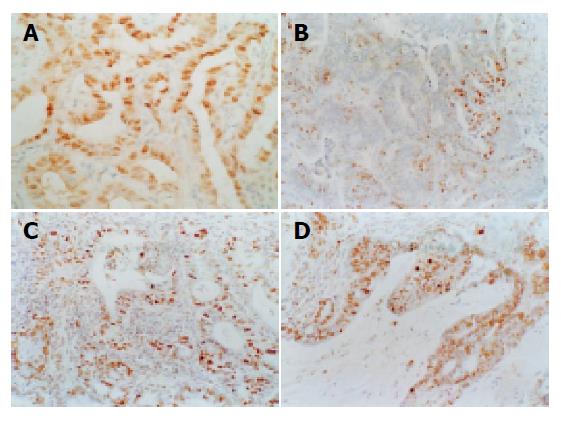
The brown-yellow positive material with the TUNEL and PCNA assays was located in the nucleolus. In normal mucosa, TUNEL-positive cells were on the epithelial layer and few PCNA-positive cells were on the bottom layer. In dysplasia, the number of TUNEL positive cells increased and distributed from the epithelium to the bottom layer. The density of distribution increased with the dysplasia grade. The number of PCNA-positive cells also increased with the dysplasia grade and the distribution of PCNA-positive cells was the same as the apoptotic cells. In the cancerous stage, the number of TUNEL-positive cells obviously decreased and distributed randomly. The number of PCNA positive cells was distinctly increased and distributed evenly (Figures 3A-3D).
Chemoprevention of malignant tumors is a field of intense interest. In China, the incidence of large intestinal cancer has been increased year after year and the patients with this cancer are tending to be younger. Therefore, safe and effective medicine needs to be identified for prevention and cure of colon carcinoma. It has been reported that non-steroidal anti-inflammatory drugs (NSAID), such as sulindac and aspirin, have some ability to prevent and cure large intestinal carcinoma[3], esophageal carcinoma and cancer of the pancreas, bladder, liver, breast and lung[3,4]. Results from our study showed that not only the incidence of large intestinal cancer in the sulindac-preventative and curative groups was lower than in model-agent group but that the grade of pathologic lesions in the sulindac-preventative and curative groups were less than in the model-agent group. The incidence of large intestinal cancer in the model-agent group was reduced 46.7-50.4% in the sulindac-preventative group and 41.1% in the sulindac-curative group. All of these results showed that sulindac had beneficial effect on colon cancer prevention and treatment. However, the exact mechanism by which sulindac prevents large intestinal cancer is unknown. Because there is more prostaglandin production from tumor tissue than normal tissue, the anti-tumor mechanism of NSAIDs has been suggested to be due to a reduction in prostaglandin formation[5-7].
In recent years, the degree of apoptotic activity has been considered to play a role in tumorigenesis[8]. The results from our study have shown that the dynamic balance between proliferation and apoptosis sustained a steady number in normal tissue which demonstrated that apoptotic cells were on the surface layer of the mucosa and formed all apoptotic cell zone with the proliferating cells located at the bottom of the mucosa forming a proliferative cells zone. The dynamic balance between cellular proliferation and apoptosis in normal tissue was destroyed in cancer tissue with an abnormal number and distribution of cellular apoptosis and proliferation. The distributive relationship between proliferation and apoptosis disappeared[9]. The results of our new study demonstrated that the density of proliferative and apoptotic cells increased gradually with the enhancing dysplasia. However, in cancer tissue the density of proliferative cells increased and the density of the apoptotic cells decreased. All the results indicate that cell proliferation and apoptosis were active in the precancerous stage but with tissue canceration the high-apoptosis cells were removed. At the same time, cells with a high-proliferative rate were saved. The ratio between apoptosis and proliferation decreased continuously. Based on these data, we proposed the concept of Selective Cell Proliferation relating to an imbalance between proliferation and apoptosis during the process of large intestinal epithelium canceration[10,11]. That is to say, there are a large number of proliferative and apoptotic cells in precancerous lesions but only those cells with a high-proliferation and a low-apoptotis are maintained so those cells with a high-apoptotic potential are diminished[12]. From this point of view, the Selective Cell Proliferation concept suggests that tumor tissue progression is a process of cellular adaptation to the environment.
In studying sulindac inhibition of large intestinal carcinogenesis in mice we have drawn the conclusion that sulindac can suppress the emergence of large intestinal cancer. In different stages of canceration, the density of apoptotic cells in the sulindac-preventative group and curative group was higher than in the model-agent group. The extent of apoptosis increased with degrees of the pathologic change and this change influenced the growth rate of the tumors, which delayed the process of tumorigenesis.
In a word, sulindac can induce cellular apoptosis causing a delay in the development of tumors. However, cancerous progression is regulated by multigenes and the exact mechanism by which sulindac induces the tumor cell apoptosis is unknown[13-15]. It is clear that the relationship between sulindac inhibition of large intestinal carcinogenesis and Selective Cell Proliferation needs further study.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Piazza GA, Rahm AK, Finn TS, Fryer BH, Li H, Stoumen AL, Pamukcu R, Ahnen DJ. Apoptosis primarily accounts for the growth-inhibitory properties of sulindac metabolites and involves a mechanism that is independent of cyclooxygenase inhibition, cell cycle arrest, and p53 induction. Cancer Res. 1997;57:2452-2459. [PubMed] |
| 2. | Duperron C, Castonguay A. Chemopreventive efficacies of aspirin and sulindac against lung tumorigenesis in A/J mice. Carcinogenesis. 1997;18:1001-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 86] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Samaha HS, Kelloff GJ, Steele V, Rao CV, Reddy BS. Modulation of apoptosis by sulindac, curcumin, phenylethyl-3-methylcaffeate, and 6-phenylhexyl isothiocyanate: apoptotic index as a biomarker in colon cancer chemoprevention and promotion. Cancer Res. 1997;57:1301-1305. [PubMed] |
| 4. | Keller JJ, Offerhaus GJ, Drillenburg P, Caspers E, Musler A, Ristimäki A, Giardiello FM. Molecular analysis of sulindac-resistant adenomas in familial adenomatous polyposis. Clin Cancer Res. 2001;7:4000-4007. [PubMed] |
| 5. | Cahlin C, Gelin J, Delbro D, Lönnroth C, Doi C, Lundholm K. Effect of cyclooxygenase and nitric oxide synthase inhibitors on tumor growth in mouse tumor models with and without cancer cachexia related to prostanoids. Cancer Res. 2000;60:1742-1749. [PubMed] |
| 6. | Sunayama K, Konno H, Nakamura T, Kashiwabara H, Shoji T, Tsuneyoshi T, Nakamura S. The role of cyclooxygenase-2 (COX-2) in two different morphological stages of intestinal polyps in APC(Delta474) knockout mice. Carcinogenesis. 2002;23:1351-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Kawada M, Seno H, Wada M, Suzuki K, Kanda N, Kayahara T, Fukui H, Sawada M, Kajiyama T, Sakai M. Cyclooxygenase-2 expression and angiogenesis in gastric hyperplastic polyp--association with polyp size. Digestion. 2003;67:20-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Bedi A, Pasricha PJ, Akhtar AJ, Barber JP, Bedi GC, Giardiello FM, Zehnbauer BA, Hamilton SR, Jones RJ. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995;55:1811-1816. [PubMed] |
| 9. | Seno H, Oshima M, Ishikawa TO, Oshima H, Takaku K, Chiba T, Narumiya S, Taketo MM. Cyclooxygenase 2- and prostaglandin E(2) receptor EP(2)-dependent angiogenesis in Apc(Delta716) mouse intestinal polyps. Cancer Res. 2002;62:506-511. [PubMed] |
| 10. | Sun BC, Zhang NX, Zhao XL, Zhang MF. The expression of cell apoptosis and the regulated gene in large cancer and adenoma. Zhonghua Binglixue Zazhi. 1997;26:137-140. |
| 11. | Sun BC, Wang L, Zhao XL, Li L, Liu YX, Yan XY, Wang X, Gu Q. The effects of the out of balance of the apoptosis and the proliferation on the large bowel carcinogenesis in mice. Zhongguo Zhongliu Linchuang. 2003;31:45-49. |
| 12. | Sun BC, Zhao XL, Yan XY, Zhang SW. Study of expression influence of sulindac to Fas and Fasl protein in cancerous lesion J. Tianjin Yiyao. 2003;31:701-704. |
| 13. | Reinacher-Schick A, Schoeneck A, Graeven U, Schwarte-Waldhoff I, Schmiegel W. Mesalazine causes a mitotic arrest and induces caspase-dependent apoptosis in colon carcinoma cells. Carcinogenesis. 2003;24:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Reddy BS, Kawamori T, Lubet RA, Steele VE, Kelloff GJ, Rao CV. Chemopreventive efficacy of sulindac sulfone against colon cancer depends on time of administration during carcinogenic process. Cancer Res. 1999;59:3387-3391. [PubMed] |
| 15. | Gardner SH, Hawcroft G, Hull MA. Effect of nonsteroidal anti-inflammatory drugs on beta-catenin protein levels and catenin-related transcription in human colorectal cancer cells. Br J Cancer. 2004;91:153-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |