Published online May 14, 2005. doi: 10.3748/wjg.v11.i18.2784
Revised: October 10, 2004
Accepted: November 29, 2004
Published online: May 14, 2005
AIM: To evaluate the efficacy of antral exfoliative cytology method in the diagnosis of Helicobacter pylori (H pylori) infection in the stomach.
METHODS: Fifty patients were submitted to upper digestive tract endoscopy due to complaints of dyspepsia. The material for exfoliative cytology was obtained by extensive brushing of the gastric antral mucosa and Papanicolaou stain was used to identify the bacteria. The authors also performed gastric biopsies to collect material for urease tests and histologic studies, with hematoxylin-eosin and fucsin stains in order to identify the microorganism. The gold standard used to detect the presence of H pylori was an analysis of the combined results from the gastric biopsies by urease test and histological method.
RESULTS: Antral exfoliative cytology method exhibited 90.3% sensitivity, 66.6% specificity, accuracy of 81.6%, positive predictive value of 82.3% and negative predictive value of 80.0%, in this population with a prevalence of 63.3%.
CONCLUSION: Antral exfoliative cytology was demonstrated to be a sensitive, accurate and easy to perform method for investigating H pylori infection in the stomach.
-
Citation: Gomes Jr CA, Catapani WR, Mader AM, Locatelli Â, Silva CB, Waisberg J. Antral exfoliative cytology for the detection of
Helicobacter pylori in the stomach. World J Gastroenterol 2005; 11(18): 2784-2788 - URL: https://www.wjgnet.com/1007-9327/full/v11/i18/2784.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i18.2784
Helicobacter pylori (H pylori) is among the pathogens that most frequently colonize the gastrointestinal tract and is found in approximately 60% of the world’s population[1].
Its distribution in the entire gastric mucosa can be focal, segmental or diffuse, and it is found within or under the layer of mucus that envelops the epithelium of gastric cells, especially in the antrum[2-4].
In broad terms, the ideal method to study H pylori infection should have high sensitivity and specificity, besides being cost-effective, easy to perform, rapid and well tolerated by patients, so that it can be repeated as necessary[5,6].
The heterogeneous distribution of H pylori in the gastric mucosa is a possible source of false-negative results[7,8]. Exfoliative cytology method utilizes a brush to collect material from the broad surface of the gastric mucosa and may prove to be a better option than other techniques that use only a small quantity of gastric fragments, collected for the urease test and histological analysis.
In view of the controversy between the various studies carried out in diverse regions of the world[6,9,10], there is still a need for continued investigations to compare endoscopic methods that are easy to perform, cost-effective and routinely used to detect the presence of H pylori in the gastric environment.
The objective of this study was to evaluate the efficacy of antral exfoliative cytology method for the diagnosis of H pylori infection of the stomach.
From May to August 2002, the authors examined 50 patients suffering from peptic gastroduodenal illness. The sample comprised seven (14%) females and 43 (86%) males, with a mean age of 42.0 years (SD 15.7; range 15-79; and median 40.0).
The inclusion criterion included free and informed consent from the patients. The exclusion criteria were: use of oral or parenteral medication for peptic disease in the previous three months; prior treatment for H pylori; pediatric patients; presence of immunodeficiency; active upper digestive bleeding; and the presence of non-peptic diseases of the upper digestive tract diagnosed during the endoscopic examination.
This investigation was conducted according to the ethical standards accepted by the Helsinki Declaration of the World Medical Association, adopted in 1964 and amended in 1996.
All patients were submitted to upper digestive endoscopies performed by the principal author. The biopsy specimens were collected using conventional biopsy forceps and the gastric brushings were obtained with protected cytological brushes (Wiltek, Medical Inc., USA).
During the endoscopic examinations, material was collected firstly by brushing the antral mucosa, after which fragments were collected from the gastric mucosa using conventional biopsy forceps for histopathological analysis. Four fragments were collected from the region of the small curvature of the antrum, at approximately 2 cm from the pylorus, and close to the incisura angularis[3,4], separating two fragments for direct detection of the presence of H pylori and histopathological analysis, and the remaining two fragments for indirect detection of the presence of H pylori by urease test. In addition, two fragments were obtained from the gastric structure, in the region of the small curvature, close to the incisura angularis, for direct detection of the presence of H pylori and histopathological analysis.
An experienced pathologist, blind to the urease test results, firstly performed the cytologic analysis and then the histological exam. The study identified H pylori bacteria with curved morphology, and S-shaped micro organisms, measuring approximately 1-3 μm.
The biopsy material was placed in bottles with buffered 10% formaldehyde for fixation and then forwarded for analysis.
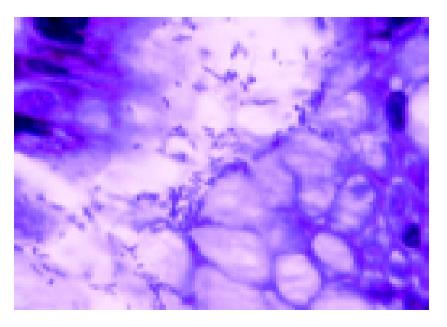
The gastric biopsies were stained with fucsin dye and the semi-quantitative evaluation of H pylori was performed according to the criteria proposed by the Sydney System[11] (Figure 1). The material from the gastric brushings was submitted to cytologic evaluation and testing for H pylori using Papanicolaou stain[12].
Cytologic screening of the gastric brushings was performed in high power fields (HPF), with 400×enlargement in an optical microscope, with semi-quantitative evaluation of the following parameters: cellularity; density of inflam-matory cells (polymorphonuclear neutrophils); and density of H pylori.
The cellularity of the smears was classified according to the presence of groupings of epithelial cells (GEC), in accordance with the following criteria: 0 (no GEC); + (1-3 GEC); ++ (4-6 GEC); and +++ (over 6 GEC)[13].
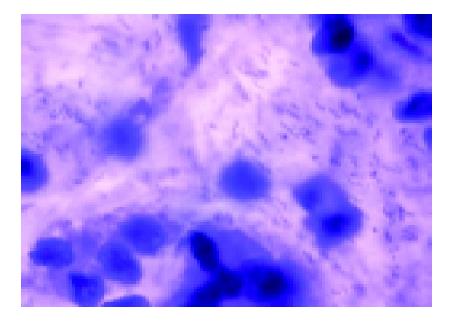
The analysis of H pylori was based on the following criteria: 0 (no bacteria detected); + (presence of sporadic bacteria); ++ (numerous bacteria in most of the fields); and +++ (clusters of bacteria in all the fields examined)[14] (Figure 2).
The density of the polymorphonuclear neutrophils (PMN) was evaluated by the examiner, according to the following parameters: absent; +/+++ (rare isolated PMNs in the smear); ++/+++ (presence of PMNs distributed among epithelial cells without forming groupings); and +++/+++ (presence of PMNs forming groupings among epithelial cells).
The urease test (Probac, Sao Paulo, Brazil) was performed by Claudio A.R. Gomes Jr. The test was recorded as positive when the color around the area of the specimen inserted changed to pink within 24 h.
To verify the agreement between the quantity of bacteria observed in the histological analysis and in the gastric brushing, and the time of any color change in the urease test, the following levels were established: level 1-characterized as no observation of bacteria in the histology and cytology, and negative urease test at 24 h; level 2-positive histology result (+), positive cytology result (+) and positive urease test at 24 h; level 3-positive histology result (++), positive cytology result (++) and positive urease test at 6 h; and level 4-positive histology result (+++), positive cytology result (+++) and positive urease test at 2 h.
The following levels were established for verifying agreement between the presence or absence of H pylori, the presence and intensity of cellularity, and the presence and intensity of inflammatory cells in the gastric brushings: level 1-neither H pylori nor inflammatory cells, absent or rare cellularity; level 2-H pylori, with discrete presence of inflammatory cells and discrete cellularity (+); level 3-H pylori, with moderate presence of inflammatory cells and moderate cellularity (++); and level 4-H pylori, with intense presence of inflammatory cells and intense cellularity (+++).
To establish the gold standard for comparison of the exfoliative gastric cytology method, the study considered patients affected by H pylori to be only those in whom the bacteria were detected by both the histology method and the urease test. The patients considered to be unaffected by the bacteria were those that tested negative in both the histology method and the urease test.
The quantitative variables were represented by their absolute (n) and relative (%) frequencies. The arithmetic mean, the median and the standard deviation of the ages were also calculated. The agreement between the different tests for the investigation of H pylori was measured by the kappa statistic (κ), which measures nonrandom agreement, with accompanying p values referring to rejection of the null hypothesis that κ = 0 (i.e., agreement due to chance alone). It was considered that values of κ>0.75 represented excellent agreement beyond chance, values of κ<0.40 showed poor agreement beyond chance, and fair to good beyond chance (“reasonable”) when 0.40≤κ≤0.75. The study also measured sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of the exfoliative cytology in relation to the gold standard, constituted by combined analyses of both the urease test and histology method results.
The calculations of the sensitivity, specificity, PPV, NPV and accuracy were based on the gold standard.
According to the criteria adopted, 49 patients were included in the make-up of the gold standard, as one patient presented a discrepancy between the urease test and histology method results. For the other agreement tests, the study used the results from all 50 patients.
In all tests, α = 0.05 was set as the rejection level for the null hypothesis, according to the standards currently adopted in biological studies.
The following illnesses were diagnosed by endoscopy among the patients included in this study: gastritis (n = 49); duodenitis (n = 9); active or healed duodenal ulcer (n = 6); hiatal hernia (n = 5); esophagitis (n = 6); gastric ulcer (n = 1); and esophageal varices (n = 1).
With regard to the histopathological analysis of the biopsies collected, this study revealed chronic gastritis in all the patients. There was active chronic gastritis in 34 patients (68%) and inactive in 16 (32%). As to the intensity of the gastritis, 11 patients (22%) presented mild gastritis, 31 (62%) moderate gastritis, and 8 (16%) severe gastritis.
Among the patients, 31 (63.26%) were infected by H pylori and 18 (36.73%) were considered uninfected, according to the criteria adopted for the study.
The cytology method confirmed 32 patients (64%) to be affected by H pylori, with 29 patients (58%) diagnosed by histological method, and 30 affected patients (60%) detected by the urease test.
When the results obtained by exfoliative cytology method were compared with those from the histology method and the urease test combined (gold standard), it was verified that exfoliative cytology presented 90.3% sensitivity, 66.6% specificity, 81.6% accuracy, PPV of 82.3% and NPV 80.0%. Accuracy represents the overall percentage of results from the exfoliative cytology tests that agree with the gold standard. Sensitivity shows the percentage of patients infected with H pylori, correctly identified as positives by the exfoliative cytology, whereas specificity indicates the percentage of patients not infected correctly identified as negatives by cytology. The predictive positive value shows the percentage of individuals with positive cytology tests that were truly infected with H pylori, whereas the negative predictive value shows the percentage of patients with negative cytology that were not in fact infected. As the positive and negative predictive values are dependent on the prevalence of the disease in the studied population, our results should be taken considering a local H pylori prevalence of 63.3% (Table 1).
| Exfoliative cytology | Histology and urease both positive | Histology and urease both negative |
| Positive | n = 28 | n = 6 |
| Negative | n = 3 | n = 12 |
When the three methods were analyzed together, the agreement between the positive and negative results of the exfoliative cytology method, urease test and histology method, as measured by the kappa statistic (κ), was reasonable (κ = 0.70) and statistically significant (P<0.001).
When the agreement was analyzed by the kappa statistic, taking two of the methods into consideration, the agreement between the urease test and the histology method was excellent (κ = 0.95); while the agreement between the urease test and the exfoliative cytology method was only reasonable (κ = 0.55). Likewise, the agreement between the exfoliative cytology method and the histology method was also considered reasonable (κ = 0.60).
According to the kappa statistic, the measurements of agreement for the quantification of H pylori by the histology and exfoliative cytology methods, and time of the color change in the urease test, were reasonable at levels 1 and 4, but no better than chance at levels 2 and 3, although the agreement was statistically significant at all levels (P<0.05) (Table 2).
| Level | Kappa value (k) | Kappa value (P) |
| 1 | 0.70 | <0.000001 |
| 2 | 0.25 | = 0.0009 |
| 3 | 0.28 | = 0.0002 |
| 4 | 0.49 | <0.000001 |
According to the kappa statistic, the results were no better than chance agreement at all levels for the presence of H pylori, inflammatory cells and cellularity in the gastric brushing.
According to the kappa statistic, there was no better than chance agreement between cellularity and presence of H pylori in the gastric brushings at levels 1-3. While at level 4, the rate of agreement was poor.
According to the kappa statistic, the agreement between the presence of inflammatory cells and H pylori in the gastric brushings was poor at all levels.
There is still no consensus regarding the diagnostic method that has to be considered as the gold standard[14], despite which histological method of analysis has been used worldwide due to its high sensitivity and the possibility it also offers for evaluating alterations in the gastric mucosa that result from the presence of these bacteria[3]. Nevertheless, urease test may be consideredas the method of choice for diagnosis of H pylori in the stomach[15]. In the present study, urease test and histology were demonstrated to have practically the same efficacy regarding the detection of H pylori in the stomach, thereby reinforcing the choice of these two exams as the gold standard for comparison with exfoliative cytology method.
Bacteria in the gastric mucosa are often present in small quantities and their irregular distribution can influence the results of exams using fragments from that site[7]. In the exfoliative cytology method used to detect H pylori in the stomach, an appropriate brush is used to obtain material from a broad area of the gastric mucosa, thereby presenting greater potential for efficacy among those cases in which the bacteria has a focalized distribution[10,16].
The present study adopted as its gold standard, the agreement between urease test and histology diagnostic methods. The exfoliative cytology method presented sensitivity of 90.3% and specificity of 66.6%. Rodríguez et al[10], compared the cytology method (Papanicolaou stain) with the histology method (hematoxylin-eosin and Warthin-Starry stain) and concluded that the former was superior for the detection of H pylori.
Paniagua et al[6], using histology as the gold standard, studied the exfoliative cytology method and found 72.0% sensitivity, 100% specificity and 77.0% accuracy, where the prevalence was 85.0%, giving an agreement of 0.76, according to the kappa statistic. In the present study, in the comparison between the results from the exfoliative cytology method and those from the histology method combined with those from the urease test, the accuracy reached 81.6%, with an underlying prevalence of 63.6%, and the kappa statistic revealed an agreement of 0.60, between the histology and cytology methods.
Dalla Libera et al[16], taking the culture of fragments from the gastric mucosa as the gold standard, demonstrated the advantages of cytology over histology for the detection of this micro-organism. In addition to greater sensitivity and specificity (100% and 92.5%, respectively) they reported that the cost and time spent by the pathologist for analyzing the material were markedly lower.
Other studies that have compared cytologic and histologic analyses for the detection of these bacteria have yielded conflicting results. De Francesco et al[17] has stated that the cytology method is more sensitive, reliable and faster than histological analysis. Ghoussoub and Lachman[13], in a study with 21 patients, concluded that histological method was superior for detection of H pylori. Mendoza et al[18], using cultures of fragments from the antral mucosa as their gold standard, obtained sensitivity of 74.4% and specificity of 92.0% with cytology, concluding that “Diff-Quik” stain makes identification of the bacteria easier than Papanicolaou technique, since it stains H pylori blue, thereby facilitating its differentiation from other bacteria and residues found on the imprint.
Carmona et al[19] compared several diagnostic methods for the detection of bacteria using biopsy and gastric brush. They concluded that exfoliative cytology, analyzed with the use of “Diff-Quik” stain, should be considered the method of choice for identification of the microorganism. The values obtained by Carmona et al[19] for sensitivity (95.23%); specificity (94.87%); PPV (96.77%), and NPV (92.50%), were higher than those found in the present study. However, when Gram’s stain was used, their values were similar to those obtained in the present study, suggesting that the Papanicolaou stain, used in this investigation, could be considered valid, with the added advantages of low cost and ease of use.
Sentürk et al[14], considering histology method as the gold standard, compared different methods for the detection of H pylori and used May-Grünwald-Giemsa stain to analyze the gastric brushings. That study revealed, using the exfoliative cytology method, a sensitivity of 87.7%, specificity of 60.7%, PPV of 85.3% and NPV of 65.4%. The results obtained by the present study showed slightly higher values than those reported by Sentürk et al[14], however, the gold standard adopted in this investigation comprised two diagnostic tests in conjunction, as opposed to the single test used by Sentürk et al[14].
This study demonstrated reasonable agreement, when none of the three methods detected the presence of H pylori or when there was a large quantity of bacteria in the specimens collected from the stomach. Using the same method (kappa statistic), for the agreement between the quantity of bacteria found in histology, cytology of the gastric brushings and the speed of the color change in the urease test. At levels 2 and 3, when the quantity of H pylori in the gastric brushing and histology was interpreted as low and moderate, respectively, the agreement between the three tests was no better than chance, demonstrating that these are cases that present evidence of greater diagnostic difficulty and disparity between the results offered by the methods used in the present study.
The low quantity of bacteria found in the gastric fragments and the prior reading could explain the false-negative results in the urease test[5]. Midolo and Marshall[8] considered that a positive urease test result requires the presence of at least 1000 bacteria in the specimens obtained from the stomach. The irregular distribution and low quantity of the microorganism in the stomach could lead to false-negative results when using the histology method[16]. The staining methods used in histologic and cytologic studies could also influence the detection of H pylori, especially in specimens with low quantities of the bacteria.
According to Debongnie et al[20], the main condition for cytology method to provide good results lies in the adequate cellularity of the gastric brushing, a factor which cannot be appropriately evaluated at the time material is being collected by cytological brush. Ghoussoub and Lachmann[13] have affirmed that the cytology method could be carried out with the collection of a sufficient amount of the mucus layer, even without adequate representation of cellularity. In this study, the kappa statistic revealed a no more than reasonable agreement only at level 4 (intense cellularity and greater density of the bacteria) in the analysis of the association between findings of cellularity and presence of H pylori. Rodríguez et al[10] have noted that the greater the cellularity of the gastric brushing, the easier it will be to detect H pylori in the material studied, a finding that was not observed in this study.
In the conditions under which this study was carried out, the results from gastric exfoliative cytology for the detection of H pylori enabled the conclusion that this method is reliable for the detection of the bacteria in the stomach, when compared with the association between the urease test and the histology method. Furthermore, exfoliative cytology, in an isolated comparison with the urease test and histology method results, presented reasonable agreement. When analyzed simultaneously for the quantification of H pylori, the exfoliative cytology method, the urease test and the histology method results were demonstrated to be reliable for confirming the absence of the microorganism in the stomach, without relation between the presence or absence of the H pylori and the intensity of the presence or absence of inflammatory and gastric epithelial cells in the exfoliative cytology.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Matysiak-Budnik T, Mégraud F. Epidemiology of Helicobacter pylori infection with special reference to professional risk. J Physiol Pharmacol. 1997;48 Suppl 4:3-17. [PubMed] |
| 2. | Hazell SL, Lee A, Brady L, Hennessy W. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J Infect Dis. 1986;153:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 291] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Genta RM, Graham DY. Comparison of biopsy sites for the histopathologic diagnosis of Helicobacter pylori: a topographic study of H. pylori density and distribution. Gastrointest Endosc. 1994;40:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 191] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Misra V, Misra S, Dwivedi M, Singh UP, Bhargava V, Gupta SC. A topographic study of Helicobacter pylori density, distribution and associated gastritis. J Gastroenterol Hepatol. 2000;15:737-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Mégraud F. Advantages and disadvantages of current diagnostic tests for the detection of Helicobacter pylori. Scand J Gastroenterol Suppl. 1996;215:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Paniagua M, Valdés L, Borbolla E, Hernández M. Exfoliative cytology for diagnosis of Helicobacter pylori. Acta Gastroenterol Latinoam. 1998;28:203-208. [PubMed] |
| 7. | Morris A, Ali MR, Brown P, Lane M, Patton K. Campylobacter pylori infection in biopsy specimens of gastric antrum: laboratory diagnosis and estimation of sampling error. J Clin Pathol. 1989;42:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Midolo P, Marshall BJ. Accurate diagnosis of Helicobacter pylori. Urease tests. Gastroenterol Clin North Am. 2000;29:871-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Pinto MM, Meriano FV, Afridi S, Taubin HL. Cytodiagnosis of Campylobacter pylori in Papanicolaou-stained imprints of gastric biopsy specimens. Acta Cytol. 1991;35:204-206. [PubMed] |
| 10. | Narváez Rodríguez I, Saez de Santamaría J, Alcalde Rubio MM, Pascasio Acevedo JM, Pabón Jaén M, Campos de Orellana AM, Soria Monge A. Cytologic brushing as a simple and rapid method in the diagnosis of Helicobacter pylori infection. Acta Cytol. 1995;39:916-919. [PubMed] |
| 11. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3553] [Article Influence: 122.5] [Reference Citation Analysis (3)] |
| 12. | Papanicolaou GN. Atlas of Exfoliative Cytology. Harvard University Press. 1954;6. |
| 13. | Ghoussoub RA, Lachman MF. A triple stain for the detection of Helicobacter pylori in gastric brushing cytology. Acta Cytol. 1997;41:1178-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Sentürk O, Cantürk Z, Erçin C, Cantürk NZ, Celebi A, Hulagu S, Paksoy N. Comparison of five detection methods for Helicobacter pylori. Acta Cytol. 2000;44:1010-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Morais M, Macedo EP, da Silva Júnior MR, Rohr MR, Ferraz ML, Castro RR, Della Libera E, Siqueira ES, Brant CQ, Ferrari Junior AP. Comparison between invasive tests for the diagnosis of Helicobacter pylori infections. Arq Gastroenterol. 1997;34:207-211. [PubMed] |
| 16. | Dalla Libera M, Pazzi P, Carli G, Contato E, Piva I, Scagliarini R, Merighi A, Ricci N, Gullini S. Brush cytology: a reliable method to detect Helicobacter pylori. J Clin Gastroenterol. 1996;22:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | De Francesco F, Nicòtina PA, Picciotto M, Martines F, Ferlazzo G, d'Aquino A. Helicobacter pylori in gastroduodenal diseases: rapid identification by endoscopic brush cytology. Diagn Cytopathol. 1993;9:430-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Mendoza ML, Martín-Rabadán P, Carrión I, Morillas JD, López-Alonso G, Diaz-Rubio M. Helicobacter pylori infection. Rapid diagnosis with brush cytology. Acta Cytol. 1993;37:181-185. [PubMed] |
| 19. | Carmona T, Muñoz E, Abad MM, Paz JI, Gómez F, Alonso MJ, Sánchez A, Bullón A. Usefulness of antral brushing samples stained with Diff-Quik in the cytologic diagnosis of Helicobacter pylori. A comparative methodologic study. Acta Cytol. 1995;39:669-672. [PubMed] |
| 20. | Debongnie JC, Delmee M, Mainguet P, Beyaert C, Haot J, Legros G. Cytology: a simple, rapid, sensitive method in the diagnosis of Helicobacter pylori. Am J Gastroenterol. 1992;87:20-23. [PubMed] |