Published online May 14, 2005. doi: 10.3748/wjg.v11.i18.2744
Revised: November 21, 2004
Accepted: December 3, 2004
Published online: May 14, 2005
AIM: To assess the clinicopathological significance of the expression of the apoptosis-inhibitory Bcl-2 protein (pBcl-2) and the apoptosis-promoting Bax protein (pBax) in human invasive ductal carcinomas (IDCs) of the pancreas.
METHODS: Fifty-nine surgical specimens of IDCs of the pancreas were stained immunohistochemically to detect pBcl-2 and pBax expressions whose correlation to tumor classification, staging, and prognosis was analyzed by univariate and multivariate analyses.
RESULTS: The expression of pBcl-2 and pBax was detected in 21 of 59 (35.6%) and in 29 of 59 (49.2%) patients with IDCs of the pancreas, respectively. Neither pBcl-2 nor pBax alone was correlated to TNM staging and differentiation degree of IDCs of the pancreas according to univariate analysis. By Mantel-Cox test, the median survival time after surgery for pBcl-2(+) and pBcl-2(-) groups were 14.3 and 7.3 mo, respectively (χ2 = 9.357, P = 0.002) and that for pBax(+) and pBax(-) groups were 12.9 and 10.2 mo, respectively (χ2 = 0.285, P>0.05). Contingency coefficient between pBcl-2 and pBax expression was 0.298, indicating that there was correlation between them (χ2 = 5.74, P<0.05). The median survival time after surgery for pBcl-2(+)pBax(+) and pBcl-2(+)pBax(-) groups were 14.3 and 14.1 mo, respectively, and that for pBcl-2(-)pBax(+) and pBcl-2(-)pBax(-) groups were 5.9 and 9.9 mo, respectively. There was a significant difference between pBcl-2(+)pBax(+) and pBcl-2(-)pBax(+) (χ2 = 5.06, P<0.05), such was the case for pBcl-2(+)pBax(+) and pBcl-2(-)pBax(-) (χ2 = 7.18, P<0.01). Cox proportional hazards model for multivariate analysis was applied, indicating that pBcl-2, TNM staging, age and pBax were high risk factors of post-surgical survival time.
CONCLUSION: Both pBcl-2 and pBax have high expression in IDCs of the pancreas, indicating that co-expression of pBcl-2 and pBax is a good indicator of favorable prognosis in IDCs of the pancreas.
- Citation: Dong M, Zhou JP, Zhang H, Guo KJ, Tian YL, Dong YT. Clinicopathological significance of Bcl-2 and Bax protein expression in human pancreatic cancer. World J Gastroenterol 2005; 11(18): 2744-2747
- URL: https://www.wjgnet.com/1007-9327/full/v11/i18/2744.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i18.2744
In North America and Europe, pancreatic cancer is the fourth and sixth leading cause of adult deaths from cancer and was responsible for close to 5% of all cancer-related deaths. Pancreatic cancer is characterized by insidious onset and rapid progress with only 3 mo of the average survival time after diagnosis. Surgery is indicated in less than 15% of the patients and the 5-year-survival rate is less than 4%. The recent research showed that expression of apoptosis-related protein is closely related to the biological characteristics of pancreatic carcinoma.
Bcl-2 gene family is the major regulator of cell apoptosis. Various homogenous or heterogeneous dimers can be formed within this gene family, enhancing or inhibiting apoptosis. For example, Bcl-2 (B-cell lymphoma-2) gene expression inhibits apoptosis, but Bax (Bcl-2 associated-x) promotes it[1]. As a 25-26 ku polypeptide, Bcl-2 protein is mainly located on nuclear membrane, endocytoplasmic reticulum and mitochondrial outer membrane. Overexpression of Bcl-2 can inhibit cell apoptosis without any impact on cell proliferation. Bcl-2 associated-x (Bax) shows 40% homology with Bcl-2 and the overexpression of this tumor-inhibiting factor may accelerate cell apoptosis. pBcl-2 and pBax have expression in various cancers but the expression in pancreatic invasive ductal carcinomas (IDCs) is still uncertain. In this experiment, we studied the expression of pBcl-2 and pBax in human IDCs of the pancreas by using immunohistochemical method (Streptavidin-biotinylated-peroxidase staining, SAB method) and investigated the correlation to the clinicopathological parameters.
Tissue samples: Fifty-nine well-documented surgical resection cases of pancreatic IDC (from May 1978 to May 1997) were obtained from our hospital. There were 19 male cases and 40 female cases. Ages of the patients ranged from 23 to 76 years (55.1±11.3 years, mean±SD). Based on TNM staging standard, 4 patients were in stage I, 8 were in stage II, 42 were in stage III, and 5 were in stage IV. Differentiation degree: 19 cases were well differentiated, 21 cases moderately differentiated and 19 cases poorly differentiated.
Antibodies: Immunostaining was performed using the streptavidin-biotin technique (SAB method). Monoclonal antibody to pBcl-2 (M124) and polyclonal antibody to pBax (A3533) were purchased from Dako Company. According to the manufacturer’s instruction, antibody dilutions at various working concentration were prepared: antibody to pBcl-2, 1:100; antibody to pBax, 1:1000.
The 4-μm sections were deparaffinized with xylene thrice for 5 min each, dehydrated in a gradient series of alcohol thrice (100%, 95% and 45% alcohol), and rinsed by PBS. Each section was treated by 0.3% glacial acetic acid, and then microwaved for antigen retrieval (800 W, 5 min), and cooled in the room temperature for 40 min. The slides were covered with 1% peroxyacetic acid for 15 min to block endogenous peroxidase activity. Non-specific binding sites were blocked by 10% normal rabbit serum for 10 min. These sections were first incubated with primary antibody for 2 h at room temperature, and then were rinsed in PBS twice. This is followed by incubation with a secondary antibody for 15 min at 37 °C and then was rinsed in PBS twice. Slides were then treated with streptoavidin-peroxidase reagent for 10 min and rinsed in PBS twice. The sections were visualized with 3,3’-diaminobenzidine (DAB) for 5 min, counterstained with hematoxylin and mounted for observation under microscope. There was slight difference between pBcl-2 and pBax staining procedure, the former adopted DAKO Company’s signal enhancement system.
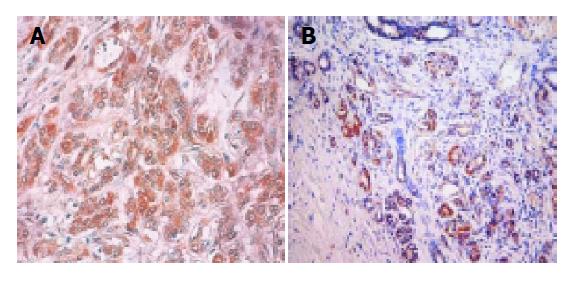
For pBcl-2, positive result was defined as 5% or more than 5% of the tumor cells showing strong brown cytoplasmic granules and for pBax, positive result was defined as 10% or more than 10% of the tumor cells showing strong brown cytoplasmic granules. Weak staining was regarded as negative.
Qualitative data analysis and correlation analysis were performed by χ2 test (or Yates’ correction test) and Pearson’s contingency coefficient, respectively. The survival curves were calculated according to Kaplan-Meier method and were compared using the Mantel-Cox test. Group comparison of univariate analysis was performed first. Furthermore, a multivariate analysis of the maximum likelihood estimate with the Cox proportional hazards model was performed to obtain the conditional risk of death due to IDC of the pancreas. All analyses were performed using the SAS 8.0 Software and a P-value of less than 0.05 was considered to be significant.
pBcl-2 was detected in 21 of 59 (35.6%) and pBax in 29 of 59 (49.2%) patients with IDCs of the pancreas (Figure 1). Contingency coefficient between pBcl-2 and pBax was 0.298, indicating that there was correlation between them (χ2 = 5.74, P<0.05). The number of pBcl-2(+)pBax(+), pBcl-2(-)pBax(-), pBcl-2(+)pBax(-) and pBcl-2(-)pBax(+) were 14, 23, 7 and 15 cases, respectively. The correlation of pBcl-2 and pBax expression to age, gender, classification, and TNM staging was shown in Table 1.
| Parameter | No. of patients | Number of pBcl-2 and pBax expressing (%) | ||||
| pBcl-2 (+) | pBcl-2 (-) | PBax (+) | PBax (-) | |||
| Overall | 59 | 21 (35.6) | 38 (64.4) | 29 (49.2) | 30 (50.8) | |
| Age (yr) | <65 | 47 | 17 (36.2) | 30 (63.8) | 26 (55.3) | 21 (44.7) |
| ≥65 | 12 | 4 (33.3) | 8 (66.7) | 3 (25) | 9 (75) | |
| Gender | Male | 38 | 14 (36.8) | 24 (63.2) | 18 (47.3) | 20 (52.7) |
| Female | 21 | 7 (33.3) | 14 (66.7) | 11 (52.4) | 10 (47.6) | |
| TNM stage | I | 4 | 1 (25) | 3 (75) | 3 (75) | 1 (25) |
| II | 8 | 5 (62.5) | 3 (37.5) | 4 (50) | 4 (50) | |
| III | 42 | 14 (33.3) | 28 (66.7) | 19 (45.2) | 23 (54.8) | |
| IV | 5 | 1 (20) | 4 (80) | 3 (60) | 2 (40) | |
| Grade | Well | 19 | 8 (42.1) | 11 (57.9) | 11 (57.9) | 8 (42.1) |
| Moderate | 21 | 8 (38.1) | 13 (61.9) | 8 (38.1) | 13 (61.9) | |
| Poor | 19 | 5 (26.3) | 14 (73.7) | 10 (52.6) | 9 (47.7) | |
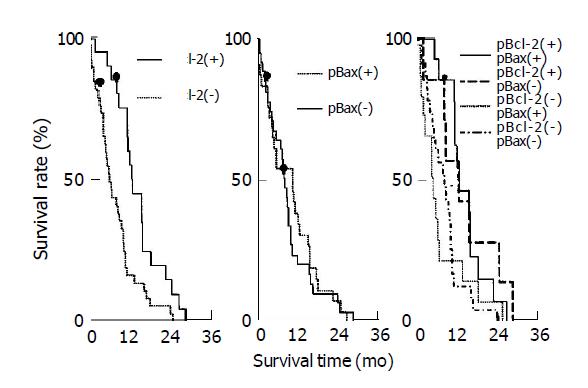
The median post-surgical survival time for pBcl-2(+) and pBcl-2(-) groups were 14.3 (95%CI: 8.1, 26.4) and 7.3 (95%CI: 2.4, 18.5) mo, respectively. Univariate analysis by Cox proportional hazards model indicated that there was a significant difference between these two groups (χ2 = 9.375, P = 0.002), indicating pBcl-2(+) strongly predicted a prolonged survival time. The median post-surgical survival time for pBax(+) and pBax(-) group were 12.9 (95%CI: 3.1, 19.8) and 10.2 (95%CI: 4.6, 14.1) mo, respectively revealing no significant difference between these two groups (χ2 = 0.285, P>0.05). The median post-surgical survival time for pBcl-2(+)pBax(+), pBcl-2(+)pBax(-), pBcl-2(-)pBax(+), pBcl-2(-)pBax(-) were 14.3 (95%CI: 8.1, 19.8), 14.1 (95%CI: 9.7, 26.4), 5.9 (95%CI: 2.4, 14.8) and 9.9 (95%CI: 4.1, 17.8) mo, respectively. All pair-wise comparisons showed that there was a significant difference between pBcl-2(+)pBax(+) and pBcl-2(-)pBax(+) (χ2 = 5.06, P<0.05), such was the case for pBcl-2(+)pBax(+) and pBcl-2(-)pBax(-) (χ2 = 7.18, P<0.01). Survival curves of different groups are shown in Figure 2. However, survival of the pBcl-2(+)pBax(+) group has no significant difference as compared to other three groups together.
Cox proportional hazards model was applied in analyzing the correlation of pBcl-2 and pBax expression to clinicopathological factors and post-surgical survival time. Multifactor analysis indicated that pBcl-2, TNM staging, age and pBax were high risk factors, while tumor site, histological grade, and gender might be low risk factors influencing post-surgical survival time (Table 2).
| Variable | Parameter estimate (SE) | Conditional risk ratio (95% CI) | P (χ2) |
| Overall pBcl-2 | -1.216 (0.340) | 0.297 (0.152-0.577) | 0.001 |
| (n = 59) TNM | 0.582 (0.246) | 1.790 (1.104-2.902) | 0.018 |
| Age (yr) | 0.037 (0.017) | 1.038 (1.004-1.073) | 0.026 |
| pBax | 0.734 (0.360) | 2.084 (1.029-4.222) | 0.041 |
| Site | -0.566 (0.487) | 0.568 (0.219-1.474) | 0.245 |
| Grade | 0.169 (0.268) | 1.184 (0.701-2.001) | 0.528 |
| Gender | 0.061 (0.352) | 1.063 (0.534-2.118) | 0.862 |
pBcl-2 is expressed in various human solid malignancies. Its expression rate is 46-77% in breast cancer, 45-86% colorectal cancer, 12% in adenocarcinoma of lung, 25% in squamous cancer of lung, and 57% in ovarian cancer[2,3]. However, there is no agreement in pBcl-2 expression of pancreatic IDCs according to different reports. Yuan and his colleagues reported that the positive rate of Bcl-2 protein was 64% in human pancreatic cancer and significantly higher along with increasing of clinical stage[4]. Makinen and his colleagues reported that pBcl-2 expression rate was 52% (34/64), and pBcl-2 expression had no correlation with tumor grading or other clinicopathological parameters[5]. Friess and his colleagues reported that Bcl-2 and Bax expression in pancreatic cancer were 28% and 83%, respectively, which were 3.7 and 5.4 times higher than normal[6]. Miyamoto and his colleagues reported that Bcl-2 and Bax expression in pancreatic cancer were 23% and 53%, respectively[7]. In our experiment, pBcl-2 and pBax were detected in 21 of 59 (35.6%) and in 29 of 59 (49.2%) cases, respectively. The disagreement in expression rate might be caused by a difference in antibody itself (different batch), the different immunohistochemical staining procedures and evaluation standard, and racial difference.
Bcl-2 gene can inhibit cell apoptosis. Although it may prevent different cell apoptosis caused by various stimuli, Bcl-2 gene cannot halt DNA injury of these apoptosis-inducing stimuli. Theoretically, pBcl-2 overexpression may shorten the survival time of the cancer patients due to the enhanced tumor cell growth caused by Bcl-2-mediated programmed cell death inhibition. But many clinical trials have proved that pBcl-2 positive patients with colorectal, breast, lung or ovarian cancer had longer survival time than the pBcl-2 negative patients[2,3,8]. The influence of pBcl-2 expression on prognosis of pancreatic IDCs is still unknown. Friess and his colleagues believed that pBcl-2 expression did not influence survival time[6]. Makinen and his colleagues reported that pBcl-2 positive patients had longer post-surgical survival time[5]. In our experiment, univariate analysis revealed that the median post-surgical survival time for pBcl-2(+) and pBcl-2(-) group were 14.3 and 7.3 mo respectively with significant difference between the two groups (χ2 = 9.357, P = 0.002), indicating pBcl-2(+) might be beneficial to assess the survival time. However, survival of the pBcl-2(+)pBax(+) group had no significant difference with the other three groups. The cause for this might be attributed to limited cases. The value of pBax expression in prediction of the prognosis is still controversial. Some scholars reported that pBax positive patient with breast or ovarian cancer had better prognosis than the pBax negative patients. pBax expression in IDCs of the pancreas varied from 53% to 100%. Friess and his colleagues believed that pBax expression played an important role in assessment of the prognosis and pBax positive patient outlasted pBax negative patients by 12 mo[6]. The median post-surgical survival time for pBax(+) and pBax(-) group were 12.9 and 10.2 mo, respectively, revealing no significant difference between the two groups (χ2 = 0.285, P>0.05). Multifactor analysis indicated that pBcl-2, TNM staging, age and pBax were high risk factors influencing post-surgical survival time, indicating that pBcl-2 expression might be related with the prognosis of the patients with pancreatic cancer.
Our further analysis on the combined expression of pBcl-2 and pBax indicated that the median post-surgical survival time for pBcl-2(+)pBax(+) and pBcl-2(-)pBax(-) were 14.3 and 9.9 mo, respectively (the latter had obviously shorter post-surgical survival time). However, there was no significant difference in post-surgical survival time between pBcl-2(-)pBax(-) and pBcl-2(-)pBax(+) group. pBcl-2 and pBax expressions were closely related. In vitro, Bcl-2 gene can inhibit cell apoptosis induced by various factors. Theoretically, pBcl-2 overexpression can inhibit cell apoptosis and promote tumor cell proliferation, which has been supported by many experiments. Bold and his colleagues found that the pancreatic cancer cell line with increased Bcl-2 expression had higher incidence of metastasis[10]. However, our study indicated that pBcl-2 positive patients with pancreatic cancer had a longer survival time. What is the explanation for the discrepancy between medical experiments and clinical practice? In vivo study on pBax reveals that Bax protein can regulate Bcl-2 activity by forming Bax-Bcl-2 heterodimers to inhibit apoptosis as well as Bax homodimers to induce apoptosis (Bcl-2 homodimers can also be formed at high concentration to inhibit apoptosis). Thus, the proportional and distributive difference in pBcl-2 and pBax expression may influence the real function of pBcl-2. On the other hand, pancreatic cancer may have distinctive biological behaviors that other cancers do not have. Our previous study has revealed that p53 expression as well as p53 gene mutation may increase the chemotherapy sensitivity of patients with pancreatic carcinoma, which disagrees with the findings in other malignancies[11].
Our study proved relatively high expression rate of pBcl-2 and pBax in IDCs of the pancreas, indicating their potential significance in assessment of prognosis. However, further study is necessary due to our limited cases.
Co-first-authors: Ming Dong and Jian-Ping Zhou
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4399] [Cited by in RCA: 4501] [Article Influence: 140.7] [Reference Citation Analysis (0)] |
| 2. | Joensuu H, Pylkkänen L, Toikkanen S. Bcl-2 protein expression and long-term survival in breast cancer. Am J Pathol. 1994;145:1191-1198. [PubMed] |
| 3. | Ofner D, Riehemann K, Maier H, Riedmann B, Nehoda H, Tötsch M, Böcker W, Jasani B, Schmid KW. Immunohistochemically detectable bcl-2 expression in colorectal carcinoma: correlation with tumour stage and patient survival. Br J Cancer. 1995;72:981-985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Yuan RW, Ding Q, Jiang HY, Qin XF, Zou SQ, Xia HS. Bcl-2, P53 protein expression and apoptosis in pancreatic cancer. Shijie Huaoren Xiaohua Zazhi. 1999;7:851-854. |
| 5. | Mäkinen K, Hakala T, Lipponen P, Alhava E, Eskelinen M. Clinical contribution of bcl-2, p53 and Ki-67 proteins in pancreatic ductal adenocarcinoma. Anticancer Res. 1998;18:615-618. [PubMed] |
| 6. | Friess H, Lu Z, Graber HU, Zimmermann A, Adler G, Korc M, Schmid RM, Büchler MW. bax, but not bcl-2, influences the prognosis of human pancreatic cancer. Gut. 1998;43:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Miyamoto Y, Hosotani R, Wada M, Lee JU, Koshiba T, Fujimoto K, Tsuji S, Nakajima S, Doi R, Kato M. Immunohistochemical analysis of Bcl-2, Bax, Bcl-X, and Mcl-1 expression in pancreatic cancers. Oncology. 1999;56:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Kapranos N, Karaiosifidi H, Valavanis C, Kouri E, Vasilaros S. Prognostic significance of apoptosis related proteins Bcl-2 and Bax in node-negative breast cancer patients. Anticancer Res. 1997;17:2499-2505. [PubMed] |
| 9. | Sarbia M, Bittinger F, Grabellus F, Verreet P, Dutkowski P, Willers R, Gabbert HE. Expression of Bax, a pro-apoptotic member of the Bcl-2 family, in esophageal squamous cell carcinoma. Int J Cancer. 1997;73:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Bold RJ, Virudachalam S, McConkey DJ. BCL2 expression correlates with metastatic potential in pancreatic cancer cell lines. Cancer. 2001;92:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Nio Y, Dong M, Uegaki K, Hirahara N, Minari Y, Sasaki S, Takamura M, Iguchi C, Tamura K. p53 expression affects the efficacy of adjuvant chemotherapy after resection of invasive ductal carcinoma of the pancreas. Anticancer Res. 1998;18:3773-3779. [PubMed] |