Published online May 14, 2005. doi: 10.3748/wjg.v11.i18.2697
Revised: August 11, 2004
Accepted: September 16, 2004
Published online: May 14, 2005
AIM: Experimental studies suggest that free radicals are involved in acid and pepsin-induced damage of esophageal mucosa. The profile and balance between free radicals and antioxidant systems in human esophagitis are unknown.
METHODS: Superoxide anion and its powerful oxidant reaction with nitric oxide (peroxynitrite) generation were determined in esophageal mucosal biopsies from 101 patients with different gastro-esophageal reflux diseases and 28 controls. Activity of both superoxide dismutase (SOD) and catalase, and reduced glutathione (GSH) levels, were also assessed. Expression of Cu,ZnSOD, MnSOD and tyrosine-nitrated MnSOD were analyzed by Western blot and/or immunohistochemistry.
RESULTS: The highest levels of superoxide anion generation were found in patients with severe lesions of esophagitis. Peroxynitrite generation was intense in Barrett’s biopsies, weaker in esophagitis and absent/weak in normal mucosa. Expression of Cu,ZnSOD and MnSOD isoforms were present in normal mucosa and increased according to the severity of the lesion, reaching the highest level in Barrett’s esophagus. However, SOD mucosal activity significantly decreased in patients with esophagitis and Barrett’s esophagus, which was, at least in part, due to nitration of its tyrosine residues. Catalase activity and GSH levels were significantly increased in mucosal specimens from patients with esophagitis and/or Barrett’s esophagus.
CONCLUSION: A decrease in SOD antioxidant activity leading to increased mucosal levels of superoxide anion and peroxynitrite radicals may contribute to the development of esophageal damage and Barrett’s esophagus in patients with gastroesophageal reflux. Administration of SOD may be a therapeutic target in the treatment of patients with esophagitis and Barrett’s esophagus.
- Citation: Jiménez P, Piazuelo E, Sánchez MT, Ortego J, Soteras F, Lanas A. Free radicals and antioxidant systems in reflux esophagitis and Barrett’s esophagus. World J Gastroenterol 2005; 11(18): 2697-2703
- URL: https://www.wjgnet.com/1007-9327/full/v11/i18/2697.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i18.2697
Gastroesophageal reflux disease (GERD) is one of the most important and frequent gastrointestinal disorders of the Western world, and may lead to esophageal cancer[1]. Although reflux of gastric acid into the esophagus is an important pathogenic factor in the development of esophagitis and Barrett’s esophagus, the severity of reflux esophagitis cannot be predicted only on the basis of acid exposure, which suggests that other factors are involved. Recent studies have shown that oxygen free radicals are involved in esophageal mucosal damage from reflux esophagitis in both experimental animal models and humans[2-9].
Reactive oxygen species (ROS) are highly reactive molecules produced during many normal biological processes. However, their synthesis is markedly increased as part of the inflammatory response, and can cause oxidative injury to cells by damaging DNA, proteins and cell membranes. Under normal conditions cells are protected by antioxidant defense systems from the toxic effects of ROS during cellular metabolism. Thus, when oxidative stress arises as a consequence of a pathological event, a defense system promotes the regulation and expression of enzyme antioxidants[10] such as superoxide dismutase (SOD), catalase and glutathione peroxidase, which constitute the principal cellular defense mechanisms against ROS.
In the esophagus, the generation of the superoxide anion as the main free radical implicated in the mucosal damage has been well established in different experimental models of acute and chronic esophagitis[2,4]. In human studies, a positive association between mucosal free radicals levels and the grade of esophagitis has been described[7,8]. However, it must be noted that the efficiency of endogenous antioxidant defense mechanism may be of critical importance in protecting against the development of esophageal mucosal injury, and although administration of various free radical scavengers has been found to prevent esophageal mucosal damage in animals[2,6], studies on the expression, activity and relevance of endogenous enzymatic antioxidant systems in the human esophageal mucosal defense are very limited[8,9]. Therefore, the aim of this study was to evaluate the profile and balance between free radicals and antioxidant systems in the spectrum of lesions associated with gastroesophageal reflux diseases, including esophagitis and Barrett’s esophagus, which we regard as the first step in order to identify future therapeutic targets.
All reagents used were purchased from Sigma Chemical Co. (St. Louis, MO) except liquid scintillation counting medium, which was purchased from Packard (Groningen, The Netherlands).
Three to five distal esophageal biopsies were taken from 3 cm above the gastroesophageal junction from consecutive patients attending the Endoscopy Unit of the Service of Digestive Diseases of the University Hospital of Zaragoza (Video Endoscope Olympus Exera). The study protocol was approved by the Institutional Review Board Committee of the University Hospital of Zaragoza and written informed consent was obtained from all patients. Patients were stratified in four groups. Group 1 (control patients): subjects with no evidence of clinical symptoms of GERD or endoscopic esophagitis who underwent upper gastrointestinal endoscopy as part of clinical evaluation for different conditions such as abdominal pain, anemia, etc. Group 2 (GERD symptoms): patients with clinical symptoms of GERD, and/or erythema but no ulcerative lesions were included in this group. Symptoms were either heartburn or regurgitation. Group 3 (esophagitis): patients with esophagitis II-IV according to the Savary-Miller classification[11]. Symptoms were either heartburn or regurgitation or chest pain. Group 4 (Barrett’s esophagus): patients with Barrett’s esophagus (mucosal length ≥3 cm). The presence of both endoscopic and histological examination of the esophageal mucosa was required to establish the diagnosis of Barrett’s esophagus[12]. In order to evaluate whether oxidative stress levels in Barrett’s epithelium was specific and associated with gastroesophageal reflux or they were intrinsic to the columnar epithelium, additional duodenal biopsies were taken in patients with/without Barrett’s esophagus.
Samples were immediately stored in Krebs-Ringer solution at 4 °C for determination of free radicals, in 10% formaldehyde for histological studies and in liquid nitrogen for Western blot analysis.
The presence of superoxide anion was evaluated by chemiluminescence according to a method described by Olyaee[7] with modifications. Biopsies were suspended in 3 mL of oxygenated Krebs-Ringer and then lucigenin (N-methylacridinium nitrate) was added at a final concentration of 0.25 mmol/L and placed in the chemiluminescence spectrophotometer where light production was measured for 2 min (1900 TR, Packard Instruments Company, Meriden, CT). At this point, SOD (200 U/mL) was added to the incubation medium and chemiluminescence levels were measured again for 2 min. Data were expressed as counts per minute (cpm) per milligram of protein. Proteins were determined by a colorimetric assay similar to Lowry assay (Bio-Rad, Hercules, CA)[13].
Total SOD activity was measured according to a method previously described by Thomas et al[14]. The assay is based on the inhibition of nitro blue tetrazolium (NBT) mediated by superoxide anion. Thus, esophageal biopsies were sonicated in Tris buffer (0.1 mol/L pH 8.1 at 4 °C) and centrifuged at 12000 g for 10 min at 4 °C. Afterwards, 200 μL of supernatant were incubated with 1.8 mL of buffer containing Na2CO3 (0.05 mol/L), xanthine (10-4 mol/L), EDTA (10-4 mol/L), and NBT (2.5×10-5 mol/L). Superoxide radical was generated by adding xanthine oxidase (200 U/L) to the reaction cuvettes and measurements were recorded at 560 nm (Shimadzu spectrophotometer, Tokyo, Japan). Results are expressed as units per milligram of protein.
Reduced glutathione (GSH) concentration was determined according to the method described by Toth et al[15], with modifications. Samples were sonicated in 1 mL of phosphate buffered saline (PBS) and centrifuged at 12000 g for 10 min at 4 °C. Ten percent metaphosphoric acid was added to 300 µL of supernatant and centrifuged. Afterwards, the supernatant was incubated with 75 µL of DNTB (5,5’-dithiobis-(2-nitrobenzoic acid)) and 0.3 mol/L Na2HPO4 (600 μL). The change of absorbance was monitored at a wavelength of 412 nm. Results are expressed as micromole per milligram of protein.
Catalase was determined according to the method described by Aebi[16]. Esophageal mucosal biopsies were sonicated in phosphate buffer (KH2PO4, 25 mmol/L) and centrifuged at 12000 g for 10 min at 4 °C. One milliliter of hydrogen peroxide (10 mmol/L) was added to 300 μL of supernatant. Absorbance decrease was read at 240 nm for 1 min. Results are expressed as units per milligram of protein.
Formalin-fixed, paraffin-embedded tissue samples were sectioned and applied to slides. Slides were subsequently deparaffinized with xylene for 15 min, hydrated gradually in a graded series of ethanol (100%, 96%, 70%, twice for 3 min in each) and washed in deionized water. Then, slides were treated with a 0.3% H2O2-methanol solution for 30 min to quench endogenous peroxidase activity and incubated for 30 min in 1.5% normal blocking serum in PBS. Immuno-histochemistry was performed using the ABC staining system. The sections were incubated with the primary antibody for 30 min at room temperature. The rabbit anti-nitrotyrosine polyclonal antibody (Chemicon International, Temecula, CA) was diluted 1:2000 and the rabbit Cu,ZnSOD polyclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was diluted 1:1000. Slides were incubated with the secondary antibody for 30 min and then, washed with three changes of PBS for 5 min each. The chromogen used was 3,3’-diaminobenzidine tetrahydrochloride. Finally, the sections were washed in deionized H2O, counterstained with Harri’s hematoxylin solution and dehydrated through ethanol (65% and 100%, twice each) and xylene (thrice). The intensity of immunohistochemistry staining was determined by semiqua-ntitative analysis and was graded blinded according to the follo-wing scale: 0: absent, 1: weak, 2: moderate, and 3: intense.
Esophageal biopsies were sonicated in PBS containing 0.1 mmol/L phenylmethyl sulfonyl fluoride, 5 μg/mL aprotinin and 1 mmol/L sodium orthovanadate. Supernatants were collected and protein concentrations were determined using a protein assay based on the Bradford method (Protein Assay Dye Reagent, Bio Rad Laboratories, München, Germany). Solubilized proteins were precleared (3 h at room temperature) with 5 μL of protein Gagarose (Roche Applied Science, Mannheim, Germany). After a brief centrifugation, the supernatants were collected in order to determine Cu,ZnSOD and MnSOD expression and the presence of tyrosine-nitrated MnSOD (22 μg/lane). In order to purify those proteins containing tyrosine-nitrated residues, we performed immunoprecipitation using a monoclonal anti-nitrotyrosine antibody. For this purpose, the supernatants were incubated (1 h at room temperature) with 10 μL of monoclonal anti-nitrotyrosine antibody conjugated to protein A agarose (Alexis Biochemicals, Lausen, Switzerland). After incubation, beads were collected by brief centrifugation, and the supernatant was carefully pipetted off. Then the beads were washed thrice with PBS. After the last wash, the beads were resuspended in 25 μL of 1×Laemmli sample buffer and boiled for 5 min. The sample was briefly centrifuged to pellet the beads, and the supernatants were immediately subjected to electrophoresis in 12% SDS polyacrylamide gel[17,18].
Western blot analysis of Cu,ZnSOD and MnSOD was performed. MnSOD was performed in both immunopre-cipitated and non-immunoprecipitated tyrosine-nitrated protein fractions. After separation by SDS/PAGE, proteins were transferred electrophoretically (100 V, 1.5 h) to PDVF membranes (Bio Rad Laboratories, München, Germany), which were blocked (4 °C, overnight) with 5% blocking agent in 50 mmol/L Tris-HCl, pH 7.4/150 mmol/L NaCl/0.05% Tween 20 (TBST). For detection of MnSOD, blots were incubated (1 h, room temperature) with a rabbit polyclonal anti-MnSOD antibody (1:1000 dilution) (Upstate, Charlottesville, VA, USA). For detection of Cu,ZnSOD, blots were incubated (1 h, room temperature) with a rabbit polyclonal anti-Cu,ZnSOD antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). After incubation with primary antibodies, membranes were washed in TBS/T, then the immunocomplexed membranes were probed (1 h, room temperature) with 1:3000 dilution of peroxidase-conjugated secondary antibody (Amersham Biosciences, Buckinghamshire, UK) in TBS with 0.1% of blocking agent. Probed membranes were washed in TBS/T and immunoreactive proteins were detected using enhanced chemiluminescence (ECL Western blotting analysis system, Amersham Biosciences).
Results from biochemical assays were expressed as the mean±SE. Values obtained from biopsies were compared between the groups by nonparametric methods using Kruskal-Wallis and Mann-Whitney tests. Descriptive statistics were used for immunohistochemical data, analyzing median, minimum, maximum and percentage. Significance between the intensity of immunohistochemistry staining and grade of esophagitis was analyzed using the Kruskal-Wallis test. A value of P<0.05 was considered statistically significant.
Patient characteristics are shown in Table 1. Age and sex of patients and controls were homogenous across the different groups. Table 1 also includes the type of treatment received by patients 30 d before the endoscopic procedure.
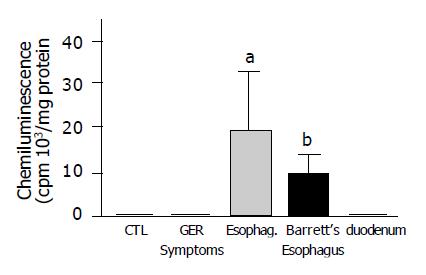
ROS were significantly increased in mucosal biopsy specimens obtained from patients with erosive esophagitis (grade II-IV) and Barrett’s esophagus. Duodenal biopsies showed similar ROS levels than esophageal biopsies of control patients (Figure 1). The addition of exogenous SOD inhibited chemiluminescence in all groups, indicating that the increase in mucosal reactive species was due to the presence of superoxide anion (data not shown).
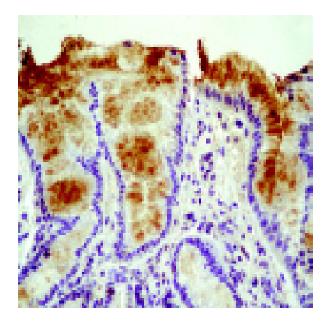
Peroxynitrite generation in the esophageal mucosa was investigated by measuring nitrotyrosine formation in esophageal biopsies by immunohistochemistry. Nitrotyrosine was detected in both the squamous and Barrett’s epithelium. Nitrotyrosine staining was intense in Barrett’s biopsies, weaker in esophagitis and absent/weak in normal esophageal mucosa (Figure 2 and Table 2).
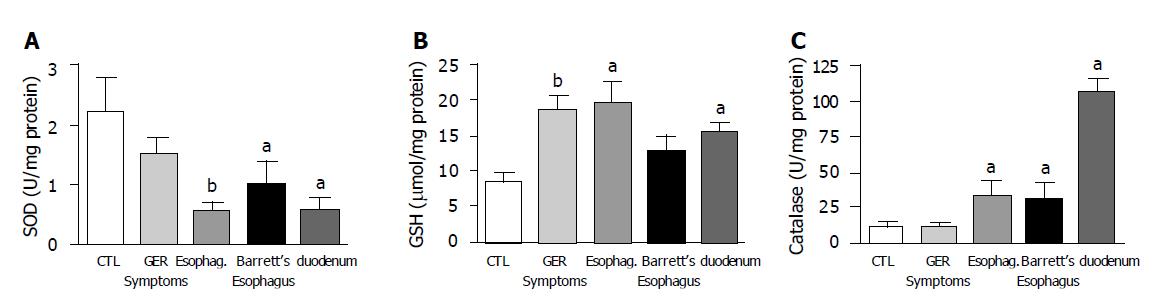
SOD activity showed a significant decrease in both biopsies taken from patients with erosive esophagitis and Barrett’s esophagus when compared to normal esophageal epithelium. The lowest SOD activity was found in biopsies with erosive esophagitis. SOD activity was significantly lower in duodenal mucosa than in normal esophagus samples (Figure 3A). GSH levels increased with esophageal mucosal damage. Significant differences were found between patients with gastroesophageal reflux as compared to normal esophagus. In duodenal mucosal specimens, values were comparable to those of Barrett’s epithelium, which were significantly higher than in normal esophageal mucosa (Figure 3B).
Catalase activity was significantly higher in patients with erosive esophagitis and Barrett’s esophagus compared to normal squamous epithelium. Duodenal mucosa also had higher values of catalase activity than the squamous epithelium (Figure 3C).
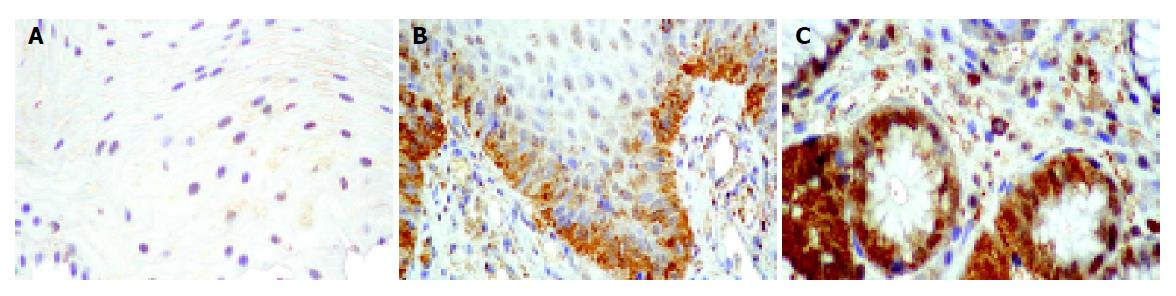
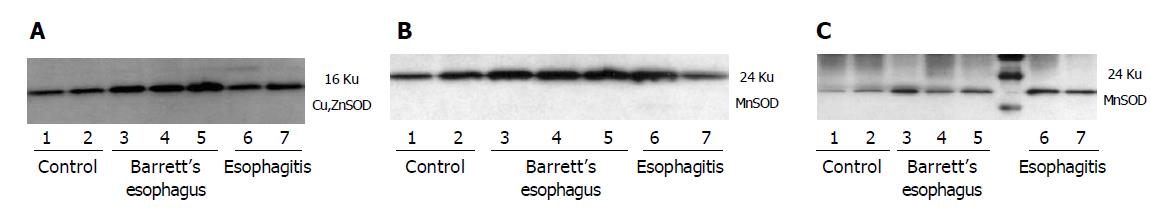
To investigate the mechanisms leading to a decrease of total SOD activity in damaged esophageal mucosa, MnSOD and Cu,ZnSOD protein expression were evaluated by immunohistochemistry and/or Western blot analysis. Furthe-rmore, nitration of MnSOD was evaluated by Western blot. By immunohistochemistry, semiquantitative evaluation revealed that Cu,ZnSOD was present in control subjects and patients with esophagitis and Barrett’s esophagus, but its expression increased according to the severity of lesion, reaching the highest level in mucosal specimens from patients with Barrett’s esophagus (Table 3 and Figure 4). In addition, Western blot analysis of Cu,ZnSOD expression in esophageal biopsies (Figure 5A) demonstrated an increase of Cu,ZnSOD expression in Barrett’s esophagus biopsies (lanes 3-5) compared to control subjects (lanes 1 and 2) and patients with esophagitis (lanes 6 and 7).
The expression of MnSOD protein was examined by Western blot. Protein extracts from biopsies were immunoblotted with a polyclonal antibody anti-MnSOD. A band at 24 ku corresponding to monomeric MnSOD was observed in all groups evaluated. Increased staining for MnSOD was observed in Barrett’s esophagus and esophagitis compared to control patients (Figure 5B). Because MnSOD is a sensitive target of tyrosine nitration, anti-MnSOD Western analysis of nitrotyrosine immunoprecipitates was performed. Immuno-blotting of nitrated protein and subsequent incubation with anti-MnSOD is present in all groups evaluated; however, the amount of nitrated protein increases in patients with esophagitis and Barrett’s esophagus compared to normal subjects (Figure 5C).
Oxidative stress is defined as an imbalance between oxidant production and the antioxidant capacity of the cell to prevent oxidative damage[19]. In the present study we have shown that esophageal mucosal damage in patients with GERD is associated with increased oxidative stress characterized by enhanced superoxide anion and peroxynitrite generation, which is accompanied by a clear pattern of antioxidant activity characterized by a profound decrease in SOD activity and an increase in GSH content and catalase activity.
In our work, we found that the main oxidant product in reflux esophagitis was superoxide anion. There was also a positive correlation between the degree of mucosal damage and levels of superoxide anion, reaching the highest values in patients with erosive esophagitis and Barrett’s esophagus. Generation of the superoxide anion as the main free radical involved in mucosal damage had been established in different experimental models of acute and chronic esophagitis[2,3,5]. The involvement of free radicals in human esophagitis has been less explored. Olyaee[7] and Westcher[8], who measured mucosal ROS concentrations by LCEL (luminol enhanced concentrations), found increased mucosal production of chemiluminescence levels in patients with esophagitis and Barrett’s esophagus.
Superoxide anion, although directly promoting inflam-mation, also has other interactions that might perpetuate the inflammatory process. The superoxide anion can be scavenged by nitric oxide (NO) and to form the powerful oxidant peroxynitrite anion (ONOO-), which would amplify the inflammatory process. Then, under pathological conditions, NO competes with endogenous SOD for superoxide anion[20]. Therefore, the next logical step in this study was to determine the presence of peroxynitrite in esophageal mucosa. This study has shown that peroxynitrite anion is formed in esophageal mucosa and that this radical seems to be particularly involved in the pathogenesis of Barrett’s esophagus. Although this aspect had not been examined in humans, these findings agree with those reported in an experimental model of esophagitis where peroxynitrite formation is a common event in the presence of excess of superoxide anion radicals[3]. Furthermore, in a rat model for esophageal adenocarcinoma associated with Barrett’s esophagus, positive nitrotyrosine staining was found, suggesting that peroxynitrite generation may contribute to the progression of esophageal cancer[21]. In conclusion, both ROS and reactive nitrogen species play a significant role in reflux esophagitis and Barrett’s esophagus.
When oxidative stress occurs, a defense system promotes the regulation and expression of several antioxidant enzymes such as SOD, catalase and glutathione peroxidase. In humans, there are three forms of SOD: cytosolic Cu,ZnSOD, mitoc-hondrial MnSOD, and extracellular SOD (ECSOD). It is well known that oxidative stress increases SOD expression[22]. In our study, Cu,ZnSOD and MnSOD were present in both control patients and patients with esophagitis or Barrett’s esophagus and its expression increased according to the severity of lesion, reaching the highest level in Barrett’s esophagus.
However, although an increase in SOD expression was observed, the activity of the total SOD was found to be dramatically decreased in esophagitis and Barrett’s esophagus compared to normal esophageal epithelium. In experimental esophagitis, superoxide anion was involved in the induction of esophagitis and SOD activity levels were negligible where mucosal damage was severe[3]. On the other hand, in the low-grade model, where superoxide anion was not a key factor, no differences in endogenous SOD activity were observed[4]. In human esophagitis, Wetscher et al[8], showed that SOD decreased with the severity of esophagitis, although in patients with Barrett’s esophagus and mild associated esophagitis high levels of esophageal mucosal SOD were found. In clear contrast with those findings, Sihvo et al[9], found no differences in SOD activity in patients with esophagitis and Barrett’s esophagus when compared to control patients (normal esophagus).
In our study, we found discrepancy between the increased expression for Cu,ZnSOD or MnSOD and the reduced activity levels (total SOD), which could be attributable to endogenous inhibitors of the SOD enzymes. It is known that peroxynitrites are potent oxidants and nitrating agents that cause depletion of cellular antioxidant defenses and inactivation of enzymes (by nitration of tyrosine residues) that may adversely affect their function[23]. Human MnSOD contains nine tyrosine residues[24]. Tyr34, which is present in the active site, appeared to be the most susceptible aminoacid to peroxynitrite-mediated nitration[24,25]. In this study, incubation of nitrotyrosine immunoprecipitates with a polyclonal antibody against MnSOD revealed that nitrated MnSOD was present and that its expression increased in patients with esophagitis compared to control patients. The nitration of MnSOD can partially contribute to a loss of enzymatic activity, since it has been described that both nitration and oxidation of critical tyrosine residues of MnSOD are required for complete inactivation of the enzyme[26]. Although human Cu,ZnSOD has no tyrosine residues in its amino acid sequence, it is probable that other amino acids (such as cysteine, tryptophan) in these enzymes are subject to modification by peroxynitrites[24,27]. The reaction of human Cu,ZnSOD with peroxynitrite may cause partial inactivation of the enzyme activity by nitration and/or oxidation of the single tryptophan 32[28]. Other sources of enzymatic inactivation may also not be excluded since SOD inactivation by hydrogen peroxide[29] and by peroxyl radicals[30] has been described.
Therefore, the net effect of reduced activity of SOD is the increase of superoxide anion concentration in the cytosol and the toxic potential of superoxide-derived oxygen radicals such as peroxynitrite, aggravating esophageal damage. In addition, we have found that this process is accompanied by additional changes in the antioxidant system which may be regarded as an adaptive response to mucosal damage in an effort to neutralize the oxidative load and it is characterized by an increase of GSH levels and catalase activity. In this study, mucosal GSH levels increased in patients with esophagitis, but they were not in the mucosa of Barrett’s esophagus. Studies regarding the role of endogenous GSH in reflux esophagitis are contradictory. Wetscher[6] and Katada[31] found an increase of esophageal tissue GSH levels in a model of acute damage in rats, whereas inhibition of esophageal damage decreased the production of GSH. These authors suggest that the increase of GSH production is a marker of prolonged oxidative stress. However, Lee et al[32], observed depletions of reduced glutathione in experimentally induced reflux esophagitis. In contrast to our findings in humans, it has been reported that GSH content was lower both in Barrett’s esophagus and esophagitis when compared to patients without symptoms or endoscopic evidence of esophageal damage[9] and when compared to normal adjacent squamous epithelium[33,34].
The GSH-dependent system is a scavenger of great importance, since it acts at different levels of the antioxidant defense system: it is a scavenger for hydrogen peroxide, peroxides and superoxide anion and it also helps to maintain the sulfhydryl groups of proteins in the reduced form for their normal function[35]. Furthermore, GSH is a substrate for the antioxidant enzyme glutathione peroxidase, which has activity as peroxynitrite reductase and therefore GSH can scavenge peroxynitrite with the formation of oxidized glutathione which is converted back to GSH. The increase of GSH content in esophageal inflammation, which was found in our study, may represent an adaptive response both to the oxidant stress in order to neutralize the oxidative load (superoxide anion and peroxynitrites), and to protect against peroxynitrite-mediated protein oxidation. The absence of increase of GSH levels in the mucosa of Barrett’s esophagus may be a consequence of the presence of the metaplastic epithelium which shows higher SOD activity and/or loss of that particular adaptive response over time.
Although other free radicals such as hydrogen peroxide have not been investigated in the present study, we cannot exclude a potential role in the pathogenesis of esophageal oxidative damage[2]. Experimental studies carried out in a model of acute esophagitis in rabbits showed that hydrogen peroxide generation was not detected in mucosal cells isolated after mucosal damage. In addition, the exogenous administration of catalase, a hydrogen peroxide scavenger, showed a trend to improvement, but it was much less effective than SOD in preventing or reducing the mucosal damage. In this work, the highest values of catalase activity were found in duodenal mucosa. However, as compared to normal esophagus, catalase activity is increased in esophagitis and Barrett’s esophagus and might play a role in the acquisition of tolerance to oxidative stress in an adaptive response of cells[10].
In conclusion, a decrease in SOD antioxidant activity may contribute to the development of esophageal damage and Barrett’s esophagus in GERD patients, leading to an increase of mucosal generation of superoxide anion and peroxynitrite radicals and the subsequent cell damage. Glutathione levels and catalase activity increase in response to this oxidative overload. We propose that administration of SOD may be a therapeutic target in the treatment of reflux esophagitis and complications as Barrett’s esophagus.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Farhadi A, Fields J, Banan A, Keshavarzian A. Reactive oxygen species: are they involved in the pathogenesis of GERD, Barrett's esophagus, and the latter's progression toward esophageal cancer? Am J Gastroenterol. 2002;97:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Naya MJ, Pereboom D, Ortego J, Alda JO, Lanas A. Superoxide anions produced by inflammatory cells play an important part in the pathogenesis of acid and pepsin induced oesophagitis in rabbits. Gut. 1997;40:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Lanas A, Soteras F, Jimenez P, Fiteni I, Piazuelo E, Royo Y, Ortego J, Iñarrea P, Esteva F. Superoxide anion and nitric oxide in high-grade esophagitis induced by acid and pepsin in rabbits. Dig Dis Sci. 2001;46:2733-2743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Soteras F, Lanas A, Fiteni I, Royo Y, Jimenez P, Iñarrea P, Ortego J, Esteva F. Nitric oxide and superoxide anion in low-grade esophagitis induced by acid and pepsin in rabbits. Dig Dis Sci. 2000;45:1802-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Wetscher GJ, Perdikis G, Kretchmar DH, Stinson RG, Bagchi D, Redmond EJ, Adrian TE, Hinder RA. Esophagitis in Sprague-Dawley rats is mediated by free radicals. Dig Dis Sci. 1995;40:1297-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Wetscher GJ, Hinder PR, Bagchi D, Perdikis G, Redmond EJ, Glaser K, Adrian TE, Hinder RA. Free radical scavengers prevent reflux esophagitis in rats. Dig Dis Sci. 1995;40:1292-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Olyaee M, Sontag S, Salman W, Schnell T, Mobarhan S, Eiznhamer D, Keshavarzian A. Mucosal reactive oxygen species production in oesophagitis and Barrett's oesophagus. Gut. 1995;37:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Wetscher GJ, Hinder RA, Bagchi D, Hinder PR, Bagchi M, Perdikis G, McGinn T. Reflux esophagitis in humans is mediated by oxygen-derived free radicals. Am J Surg. 1995;170:552-556; discussion 556-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Sihvo EI, Salminen JT, Rantanen TK, Rämö OJ, Ahotupa M, Färkkilä M, Auvinen MI, Salo JA. Oxidative stress has a role in malignant transformation in Barrett's oesophagus. Int J Cancer. 2002;102:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Matés JM, Pérez-Gómez C, Núñez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32:595-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 1263] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 11. | Savary M, Miller G. The Esophagus. Handbook and Atlas of Endoscopy. Solothurn, Switzerland: Gassmann AG 1978; 135-139. |
| 12. | Smyrk TC. Histology in the diagnosis of foregut disease. In: Hinder RA, Nyus LM, eds. Problems in General Surgery. Test of foregut function, Vol 9. Philadelphia PA JB Lippincott. 1992;14-38. |
| 13. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 14. | Thomas RM, Fang S, Leichus LS, Oberley LW, Christensen J, Murray JA, Ledlow A, Conklin JL. Antioxidant enzymes in intramural nerves of the opossum esophagus. Am J Physiol. 1996;270:G136-G142. [PubMed] |
| 15. | Toth KM, Berger EM, Beehler CJ, Repine JE. Erythrocytes from cigarette smokers contain more glutathione and catalase and protect endothelial cells from hydrogen peroxide better than do erythrocytes from nonsmokers. Am Rev Respir Dis. 1986;134:281-284. [PubMed] |
| 16. | Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15211] [Cited by in RCA: 15197] [Article Influence: 370.7] [Reference Citation Analysis (0)] |
| 17. | Pittman KM, MacMillan-Crow LA, Peters BP, Allen JB. Nitration of manganese superoxide dismutase during ocular inflammation. Exp Eye Res. 2002;74:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci USA. 1996;93:11853-11858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 607] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 19. | Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005-L1028. [PubMed] |
| 20. | Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424-C1437. [PubMed] |
| 21. | Goldstein SR, Yang GY, Chen X, Curtis SK, Yang CS. Studies of iron deposits, inducible nitric oxide synthase and nitrotyrosine in a rat model for esophageal adenocarcinoma. Carcinogenesis. 1998;19:1445-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Matés JM. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology. 2000;153:83-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 982] [Cited by in RCA: 948] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 23. | Gow AJ, Duran D, Malcolm S, Ischiropoulos H. Effects of peroxynitrite-induced protein modifications on tyrosine phosphorylation and degradation. FEBS Lett. 1996;385:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 345] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Macmillan-Crow LA, Cruthirds DL. Invited review: manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 276] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 25. | Yamakura F, Taka H, Fujimura T, Murayama K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J Biol Chem. 1998;273:14085-14089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 341] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 26. | MacMillan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry. 1998;37:1613-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 404] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 27. | Yamakura F, Matsumoto T, Fujimura T, Taka H, Murayama K, Imai T, Uchida K. Modification of a single tryptophan residue in human Cu,Zn-superoxide dismutase by peroxynitrite in the presence of bicarbonate. Biochim Biophys Acta. 2001;1548:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Ischiropoulos H, al-Mehdi AB. Peroxynitrite-mediated oxidative protein modifications. FEBS Lett. 1995;364:279-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 467] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 29. | Hodgson EK, Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: chemiluminescence and peroxidation. Biochemistry. 1975;14:5299-5303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 181] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Escobar JA, Rubio MA, Lissi EA. Sod and catalase inactivation by singlet oxygen and peroxyl radicals. Free Radic Biol Med. 1996;20:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 224] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 31. | Katada N, Hinder RA, Smyrk TC, Hiki Y, Kakita A. Duodenoesophageal reflux induces apoptosis in rat esophageal epithelium. Dig Dis Sci. 1999;44:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Oh TY, Lee JS, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Cho SW, Hahm KB. Oxidative damages are critical in pathogenesis of reflux esophagitis: implication of antioxidants in its treatment. Free Radic Biol Med. 2001;30:905-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Peters WH, Roelofs HM, Hectors MP, Nagengast FM, Jansen JB. Glutathione and glutathione S-transferases in Barrett's epithelium. Br J Cancer. 1993;67:1413-1417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | van Lieshout EM, Tiemessen DM, Witteman BJ, Jansen JB, Peters WH. Low glutathione and glutathione S-transferase levels in Barrett's esophagus as compared to normal esophageal epithelium. Jpn J Cancer Res. 1999;90:81-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Pastore A, Federici G, Bertini E, Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta. 2003;333:19-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 777] [Article Influence: 35.3] [Reference Citation Analysis (0)] |