Published online May 7, 2005. doi: 10.3748/wjg.v11.i17.2579
Revised: November 3, 2004
Accepted: November 24, 2004
Published online: May 7, 2005
AIM: To explore the expression of apoptosis-regulating genes C-jun and Bcl-XL after normothermic liver ischemic preconditioning and its protective effect on hepatocytes in the rat.
METHODS: Wistar rats are randomly divided into sham operation group (S group, n = 10), ischemic reperfusion group (IR group, n = 10) and ischemic preconditioning group (IP group, n = 10). After dissection of the hepatoduodenal ligament in S group, and after 30-min reperfusion in IR group and in IP group, the samples of liver tissue were taken for studying the hepatocellular apoptosis, the expressions of C-jun mRNA, Bcl-XL mRNA and their proteins, and morphologic changes at 0, 3, 6, 20 h. Meanwhile the venous blood samples were drawn at 3, 6 and 20 h for testing ALT, AST and LDH.
RESULTS: The levels of ALT, AST and LDH in IR group and IP group were significantly higher than those in S group. Hepatocellular apoptosis was significantly increased in both IR group and IP group, especially in IR group. Expressions of C-jun mRNA and protein were significantly increased in IR group compared with those in both IP group and S group, but no significant difference between IP group and S group (P>0.05). Expressions of Bcl-XL mRNA and protein in IR group and S group were not significant (P>0.05), but were significantly increased in IP group compared with those in both S group and IR group. Patch necrosis of hepatocytes because of severe injury could be seen in IR group microscopically, and the ultrastructural changes were irreversible. Meanwhile in IP group, no hepatocellular necrosis occurred, and the ultrastructural changes were reversible because of mild injury.
CONCLUSION: (1) IP can protect the rat liver from normothermic IR injury by modulation of the expression of apoptosis-regulating genes C-jun and Bcl-XL; (2) IR injury may activate the apoptosis of hepatocytes by increasing the expression of apoptosis-inducing gene C-jun; (3) IP may prohibit the apoptosis of hepatocytes by increasing the expression of apoptosis-inhibitory gene Bcl-XL.
- Citation: Hu GH, Lü XS. Effect of normothermic liver ischemic preconditioning on the expression of apoptosis-regulating genes C-jun and Bcl-XL in rats. World J Gastroenterol 2005; 11(17): 2579-2582
- URL: https://www.wjgnet.com/1007-9327/full/v11/i17/2579.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i17.2579
Temporary hepatic inflow occlusion, which is a practical procedure during liver surgery, can reduce the intraoperative bleeding, but may cause ischemic reperfusion (IR) injury and increase hepatocellular apoptosis as well[1]. A single or multiple brief periods (minutes) ischemia and reperfusion render tissue or organ resistance to a subsequent sustained ischemic insult. This phenomenon called ischemic preconditioning (IP), which was first discovered by Murry et al[2], in 1986, has been subsequently documented on the protective effect in a variety of organs including the myocardium[3], brain[4], kidney[5], small intestine[6] and skeletal muscle[7]. Recently, experimental study and clinical trial have suggested that IP can protect the liver from IR injury[8], but the mechanisms are still unclear. It is a new area that the study of the effect of normothermic liver ischemic preconditioning on the expression of apoptosis-regulating genes. The purpose of the research is to study the characteristics of the expression of apoptosis-regulating genes C-jun and Bcl-XL and the relationship between regulation of hepatocellular apoptosis and its protective effect on hepatocytes.
Healthy male Wistar rats, weighing 250-300 g, were randomly divided into three groups (10 in each group), which were sham operation group (S group): the liver was not subjected to ischemia or IP; IR group: the liver was subjected to 30-min ischemia and 30-min reperfusion; and ischemic preconditioning group IP group: the liver underwent 30-min ischemia and 30-min reperfusion after 5-min ischemia followed by 5-min reperfusion.
The animal models of IR and IP prepared in Wistar rats, fasting for 12 h were anesthetized by injection of the peritoneal cavity with 3% sodium pentobarbital (30 mg/kg) followed by intramuscular injection with heparin (100/μg·kg) to prevent clotting of blood after long periods of ischemia. A laparotomy was performed to expose the liver and the hepatoduodenal ligament. Total hepatic ischemia was performed by clamping the hepatoduodenal ligament with microvascular clamp followed by reperfusion after removing the clamp. Two microliters of blood samples were drawn from inferior vena cava at 3, 6 and 20 h after dissection of the hepatoduodenal ligament in S group, and after 30-min reperfusion in both IR group and IP group, for testing the marker enzymes of liver damage (ALT, AST and LDH). Meanwhile the samples of liver tissue (0.5 cm×0.5 cm×0.5 cm in size) for fresh sections (4-mm thick) were taken from the left lobes for studying the hepatocellular apoptosis with TUNEL; the expressions of C-jun, Bcl-XL mRNA with nucleic acid in situ hybridization; C-jun, Bcl-XL proteins with immunohistochemistry staining and morphologic changes with microscopy and electron microscopy at 0, 1, 3, 6 and 20 h. Finger compression was applied to stop bleeding after drawing blood and taking liver tissue. 0.9% NS of 2 mL was transfused into the peritoneal cavity at 1, 3, 6 h. The abdomen of the rats, which were returned to their cages after the suture of the abdominal wall at 6 h, were reopened at 20 h.
Kits for ALT, AST and LDH were purchased from Centronic Company (Germany). Kits for in situ hepatocellular apoptosis detection, C-jun and Bcl-XL mRNA in situ hybridization detection and C-jun and Bcl-XL protein immunohistochemistry staining were all purchased from Sigma Company (USA).
Data were expressed as mean±SE. Group comparisons were performed by ANOVA with multiple comparisons or t test when appropriate. A difference of P<0.05 was considered significant. All statistics were accomplished via software SPSS10.0 for Windows.
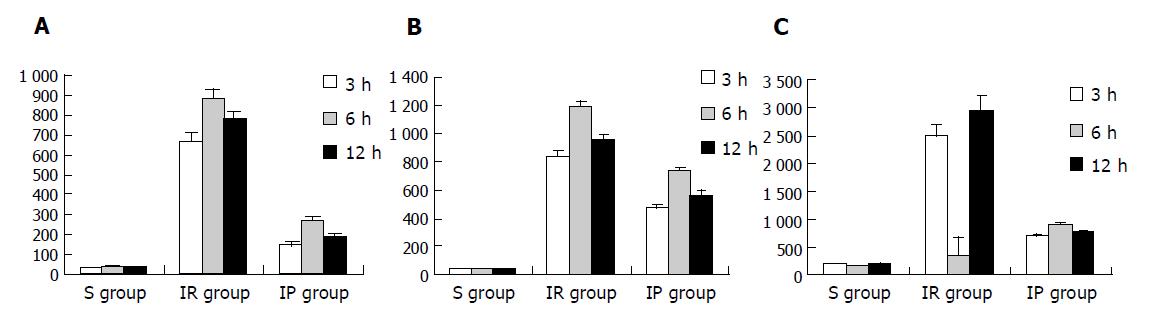
The values of these enzymes in IR group and IP group were significantly higher than those in S group (P<0.01) at the same time points. The values in IR group, with the peak level at 6-h point, were significantly higher than those in IP group (P<0.01) at the same time points (Figures 1A-1C).
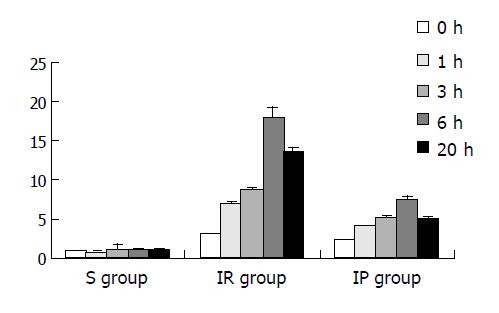
Apoptosis was rarely seen in S group, but the apoptosis index (AI) of hepatocytes was significantly increased in both IR group and IP group (P<0.01), especially in IR group (Figure 2).
Expressions of C-jun mRNA and protein were significantly increased in IR group but not significant in both S group and IR group. Compared with those in both S group and IP group, the expressions of C-jun were significantly increased at 3, 6, 20 h (P<0.05 or P<0.01) (Tables 1 and 2).
Expressions of Bcl-XL mRNA and protein were significant in IP group but not significant in both S group and IR group. Compared with those in both S group and IR group, the expressions of Bcl-XL in IP group were significantly increased at 3, 6, 20 h (P<0.05 or P<0.01) (Tables 3 and 4).
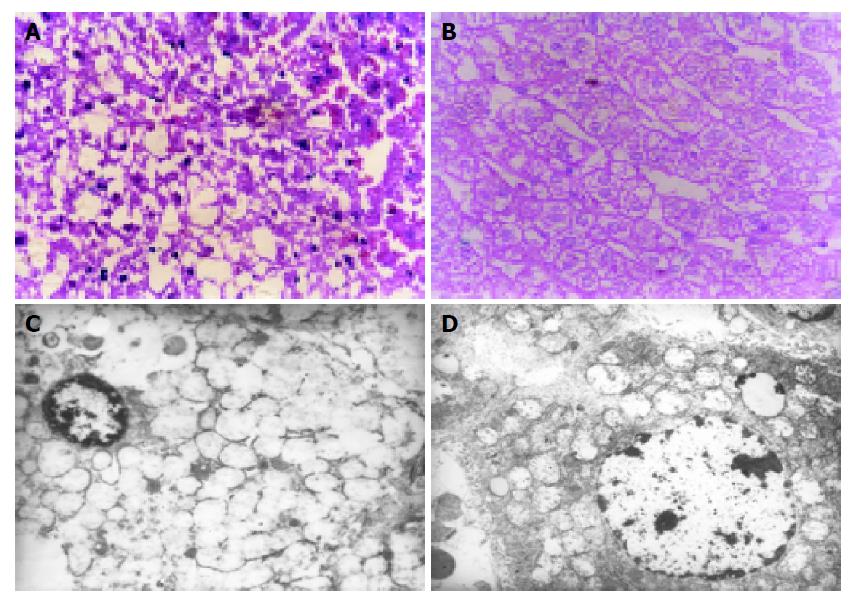
Patch necrosis of hepatocytes could be seen in IR group microscopically, and the ultrastructural changes on electron microscopy were irreversible because of severe injury, while in IP group there was no hepatocellular necrosis, and the ultrastructural changes were reversible because of mild injury (Figures 3A-3D).
Apoptosis (programmed cell death), a form of cell death, is characterized by DNA fragmentation, which is caused by the activation of endonuclease, and is a basic mechanism to maintain the homeostasis. Abnormal cellular apoptosis, which is increased or decreased, may lead to some disorders. Yadav et al[9], found that the presence of hepatocellular apoptosis played an ultimate role in the IR injury of liver. Sasaki et al[10], revealed that the rats’ hepatocellular apoptosis were significantly increased when the liver IR injury occurred. Some authors[11] also showed that IP could reduce the degree of liver IR injury.
The present study demonstrated that the hepatocellular apoptosis of rats and concentrations of AST, ALT, LDH in IR group were significantly elevated compared with those in S group, which were greatly elevated at 3-h point, reaching peak value at 6-h point and then significantly reduced at 20-h point after undergoing 30-min ischemia and 30-min reperfusion. Correspondent morphologic changes of damages in both liver tissue and liver ultrastructure could be seen microscopically and electron-microscopically. Meanwhile, we also revealed that AI, values of the marker enzymes of liver damage (ALT, AST and LDH) and morphologic changes were much improved in IP group compared with IR group. Hence, our experiment confirmed the theory that IP could diminish the degree of liver IR injury.
Although it is clear that IP exerts beneficial effects in IR, the mechanisms underlying the protective actions of IP remain uncertain. However, the bulk of the available experimental evidence suggested that the beneficial effects of IP involved adenosine production during the period of preconditioning ischemia and of reperfusion after prolonged ischemia. Some authors[12] found that adenosine served as a regulator of genes to suppress cellular apoptosis and produced protective role in IP. Our research, using the methods of nucleic acid in situ hybridization, attempted to explore the expressions of apoptosis-inducing gene C-jun and apoptosis-inhibitory gene Bcl-XL in Wistar rats during IR and IP, and found that the expression of C-jun mRNA in IR group was significantly increased and reached its peak at 3- to 6-h point. Similar findings were the expression of C-jun protein, by using the test of immunohistochemistry. These data suggested that enhanced expression of C-jun during IR might induce hepatocellular apoptosis and cause hepatocellular damage based on the aspects of AI, values of marker enzymes of liver damage and morphologic changes at the same time points. In IP group, the expressions of Bcl-XL mRNA and protein were significantly increased and reached the peaks at 6-h point with the improvement of hepatocellular apoptosis, marker enzymes of liver damage and morphology. These data suggested that the protective function of IP on IR injury was closely associated with the expression of apoptosis-inhibitory gene Bcl-XL.
Our study also demonstrated that IP protected liver IR injury by the two-way regulations through hepatocellular apoptosis-inducing gene and apoptosis-inhibitory gene. Several studies have demonstrated that the sustained expression of C-jun, which belongs to the immediate-early gene, are involved in the initiation and regulation of hepatocellular apoptosis and C-jun protein may serve as a transcriptional regulator to modulate the production of apoptosis. However, the delayed-early gene Bcl-XL has been shown to suppress apoptosis in a variety of models and Bcl-XL protein may prevent apoptosis by binding proapoptotic molecules by functioning as an ion channel, or altering mitochondrial function.
In conclusion, IP can protect the rat liver from normothermic IR injury by modulation of the expression of apoptosis-regulating genes C-jun and Bcl-XL. IR injury may activate the apoptosis of hepatocytes by increasing the expression of apoptosis-inducing gene C-jun. IP may prohibit the apoptosis of hepatocytes by increasing the expression of apoptosis-inhibitory gene Bcl-XL. We hope the results of our research will provide some new ideas on IP protecting liver IR injury for clinicians.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Shimamatsu K, Wanless IR. Role of ischemia in causing apoptosis, atrophy, and nodular hyperplasia in human liver. Hepatology. 1997;26:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 87] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5406] [Cited by in RCA: 5542] [Article Influence: 142.1] [Reference Citation Analysis (0)] |
| 3. | Li YW, Whittaker P, Kloner RA. The transient nature of the effect of ischemic preconditioning on myocardial infarct size and ventricular arrhythmia. Am Heart J. 1992;123:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 161] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc Natl Acad Sci USA. 1995;92:4666-4670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 428] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Turman MA, Bates CM. Susceptibility of human proximal tubular cells to hypoxia: effect of hypoxic preconditioning and comparison to glomerular cells. Ren Fail. 1997;19:47-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Akimitsu T, Gute DC, Korthuis RJ. Ischemic preconditioning attenuates postischemic leukocyte adhesion and emigration. Am J Physiol. 1996;271:H2052-H2059. [PubMed] |
| 7. | Pang CY, Yang RZ, Zhong A, Xu N, Boyd B, Forrest CR. Acute ischaemic preconditioning protects against skeletal muscle infarction in the pig. Cardiovasc Res. 1995;29:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Yin DP, Sankary HN, Chong AS, Ma LL, Shen J, Foster P, Williams JW. Protective effect of ischemic preconditioning on liver preservation-reperfusion injury in rats. Transplantation. 1998;66:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 151] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Yadav SS, Sindram D, Perry DK, Clavien PA. Ischemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatology. 1999;30:1223-1231. [PubMed] |
| 10. | Sasaki H, Matsuno T, Tanaka N, Orita K. Activation of apoptosis during the reperfusion phase after rat liver ischemia. Transplant Proc. 1996;28:1908-1909. [PubMed] |
| 11. | Kume M, Yamamoto Y, Saad S, Gomi T, Kimoto S, Shimabukuro T, Yagi T, Nakagami M, Takada Y, Morimoto T. Ischemic preconditioning of the liver in rats: implications of heat shock protein induction to increase tolerance of ischemia-reperfusion injury. J Lab Clin Med. 1996;128:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Lee HT. Mechanisms of ischemic preconditioning and clinical implications for multiorgan ischemic-reperfusion injury. J Cardiothorac Vasc Anesth. 1999;13:78-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |