INTRODUCTION
Though a lot of investigations were carried out on the function of α-fetoprotein (AFP), the biological role of AFP is still indistinct so far. Because the number and sequence of amino acid of AFP are very similar to those of human serum albumin (HSA), it is thought that the biological function of AFP just like HSA, plays an important role in transporting materials and regulating osmotic pressure in human blood. During the course of embryo development, the serum concentration of AFP is higher (3 g/L). The concentration of AFP falls down to a low level that it is hard to detect (<20 ng/L) when 2 years birth after. However, the expressed quantities of AFP would rise abnormally when an individual suffers from liver tumor or benign hepatic growth[1,2]. Therefore, it is reasonable to imagine that the high exp-ression of AFP is related to the proliferation of tumor or normal cells. Previous researches have shown that AFP could promote the proliferation of different types of tumor or normal cells when a sole administration or in combination with other growth factors[3,8]. Since AFP is a kind of biological macromolecule (MW: 69 ku), it is impossible to enter cells directly to regulate biological responses of the cells. Our research revealed that AFP could regulate cell growth was mediated by its receptor, which exists on the membrane of cells. Some studies also showed that AFP could inhibit immune responses mediated by its receptors[2,9,10]. The cells secrete plenty of AFP during the development of liver cancer. The potential role of AFP in the proliferation of tumor cells is still unclear though AFP has been thought as an important marker of hepatoma. It was traditionally considered that AFP was a substance accompanied with the development of liver cancer, so it was regarded as a diagnostic standard of liver cancer. However, recent studies indicated that AFP was a kind of protein with some biological activities, such as promoting the growth of cells and escaping from the host’s immune monitor of liver cancer cells in vivo. Some proteins, such as Fas as well as its natural ligand (FasL protein) and TRAIL as well as TRAILR play an important role in escaping from immune surveillance of liver cancer cells in vivo[11-14]. In the present investigation, we observed the influence of AFP on the expression of TRAIL/TRAILR or Fas/FasL and explored the possible mechanism of AFP in the growth and immune escape of liver cancer cells.
MATERIALS AND METHODS
Materials
Bel 7402 cells and Jurkat cells were presented by the Depar-tment of Cell Biology, Peking University Health Science Center (PR China). Purified AFP was purchased from Sigma (USA). Monoclonal antibody against AFP (anti-AFP) used to block AFP was offered by the Department of Biochemistry and Molecular Biology, Peking University Health Science Center (PR China). HSA and RPMI-1640 were purchased from Gibco (USA). Monoclonal antibodies against Fas and FasL were purchased from Santa Cruz (USA). Total RNA extract reagent kit and the random primer-a-gene labeling system reagent kit were products from Promage Company (Madison, WI, USA). α-32P-dCTP was bought from Yahui Biological Engineering Company (Beijing, China). TRAIL, TRAILR (DR4) and β-actin cDNA probe were presented by the Department of Endocrinology, Northwestern University (Chicago, USA). Salmon fish sperm DNA, fraction V of bovine serum albumin (BSA) and Ficoll-400 were purchased from the Jingke Chemical Reagents Company (Beijing, PR China).
Methods
Cell culture Bel7402 cells and Jurkat cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) at 37 °C in a humidified atmosphere of 50 mL/L CO2. Bel7402 cells were digested and scattered by using 0.25% trypsin containing 0.02% EDTA, and then continuously cultured. The cultured medium was replaced at every 24 h.
Northern blot analysis of TRAIL and TRAILR expression Cells were treated with AFP (20 mg/L), anti-AFP (40 mg/L) or AFP (20 mg/L) plus anti-AFP (40 mg/L) for 12 h. Total cellular RNA was isolated from Bel 7402 cells and Jurkat cells with the TRIzol reagent kit according to the manufacturer’s protocol. RNA (10-20 μg) was isolated by electrophoresis through a 1% formaldehyde agarose gel, and transferred (in 20×SSC) onto the nitrocellulose membranes (Millipore Corporation Bedford, MA, USA) with a standard procedure[15]. The membranes were hybridized with α-32P labeled probes and then exposed to X-ray films at -80 °C.
Western blot detected the expression of Fas and FasL Cells were treated with either AFP (20 mg/L), HSA (20 mg/L), anti-AFP (40 mg/L) or AFP (20 mg/L) plus anti-AFP (40 mg/L) for 24 h. After washing thrice with PBS (pH 7.4, 0.15 mol/L), the cells in each group were lyzed with 10 µL of lysis buffer containing 0.2% Triton X-100, 500 mmol/L NaCl, 500 mmol/L sucrose, 1.0 mmol/L EDTA, 0.15 mmol/L spermine, 0.5 mmol/L spermidine, 10 mmol/L HEPES (pH 8.0), 200 µmol/L phenylmethylsulfonyl fluoride, 2.0 mg/L leupeptin, 2.0 mg/L pepstatin, 24000 IU/L aprotinin and 7.0 mmol/L β-mercaptoethanol. Forty or twenty micrograms of proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto the PVDF membrane for immunodetection. Molecular weight markers (Sigma) verified the correct locations of visualized bands. The membranes were blocked with 50 g/L nonfat milk in PBS-Tween, then probed with anti-Fas or anti-FasL followed by second antibody (goat anti-rabbit IgG-horse radish peroxidase). Immunoreactive proteins were detected using Western blotting chemiluminescence luminol reagent developing systems. The results of Western blot were analyzed by a standard procedure[15].
Statistical analysis
Data were analyzed by Student’s t test and expressed as mean±SD based on three independent experiments.
RESULTS
Influence of AFP on the expression of Fas and FasL protein in human Bel 7402 cells and Jurkat cells
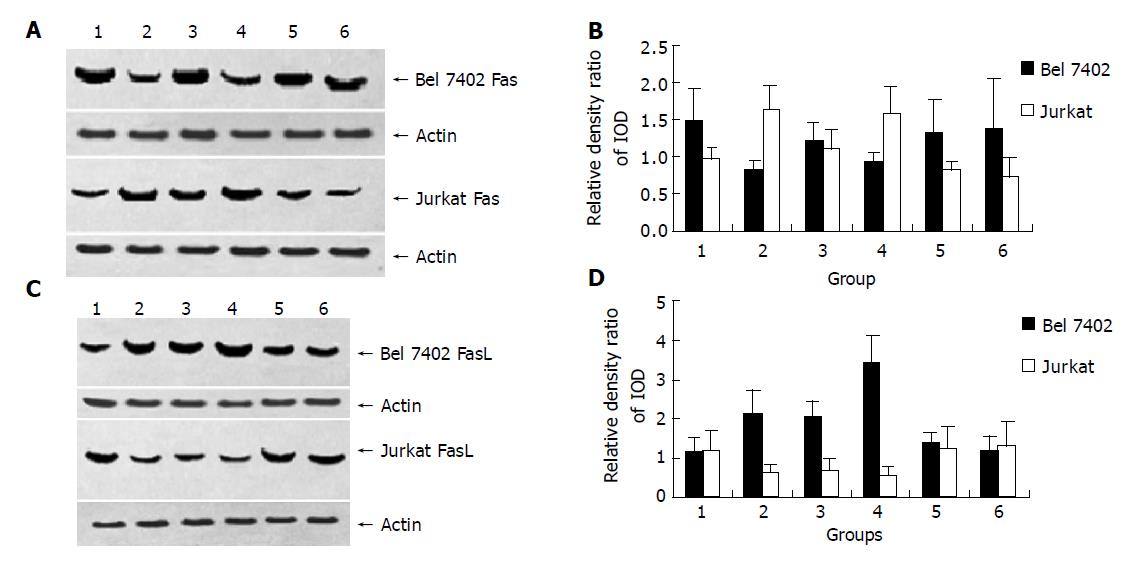
After being treated with AFP (20 mg/L), anti-AFP (40 mg/L), AFP (20 mg/L) plus anti-AFP (40 mg/L) and HSA (20 mg/L) for 24 h, AFP promoted Bel 7402 cells to express FasL and inhibit Fas protein express significantly. For Jurkat cells, AFP suppressed the expression of FasL protein, but stimulated the expression of Fas protein. When Bel7402 cells and Jurkat cells were co-incubated, AFP co-operated with Bel7402 cells to inhibit Jurkat cells to express FasL protein. HSA and anti-AFP had no significant influence on the expression of Fas and FasL protein in the two types of cells, but anti-AFP had a role in blocking the function of AFP (Figures 1 and 2).
Figure 1 Influence of AFP (20 mg/L), anti-AFP (40 mg/L), AFP (20 mg/L) plus anti-AFP (40 mg/L) and HSA (20 mg/L) on the expression of Fas and FasL protein in Bel7402 cells and Jurkat cells.
A, C: Western blot of Fas and FasL protein of Bel7402 cells or of Jurkat cells; B, D: Quantitative analysis of IOD of Fas/IOD of actin, the columns represent mean±SD.
Figure 2 Effect of AFP (20 mg/L), anti-AFP (40 mg/L) and AFP (20 mg/L) plus anti-AFP (40 mg/L) on the expression of Fas and FasL protein in Bel7402 cells or in Jurkat when the cells were cultured individually or co-cultured.
A and C: Western blot analysis of Fas and FasL protein of Bel7402 cells or of Jurkat cells; B and D: Quantitative analysis IOD of Fas/IOD of actin, the columns represent mean±SD.
Figure 1, Lane 1: Control group; lane 2: AFP treated groups; lane 3: Anti-AFP (40 mg/L) treated groups; lane 4: AFP (20 mg/L) plus Anti-AFP (40 mg/L) treated groups; lane 5: HSA (20 mg/L) treated groups.
Figure 2, Lane 1: Control groups (cells were cultured individually, respectively); lane 2: AFP treated groups (cells were cultured individually, respectively); lane 3: Cells were co-cultured groups; lane 4: Cells were co-cultured and treated with AFP group; lane 5: Cells were co-cultured and treated with anti-AFP group; lane 6: Cells were co-cultured and treated with AFP plus anti-AFP group.
Effects of AFP on the expression of TRAIL and TRAILR mRNA of human hepatoma Bel 7402 cells and Jurkat cells
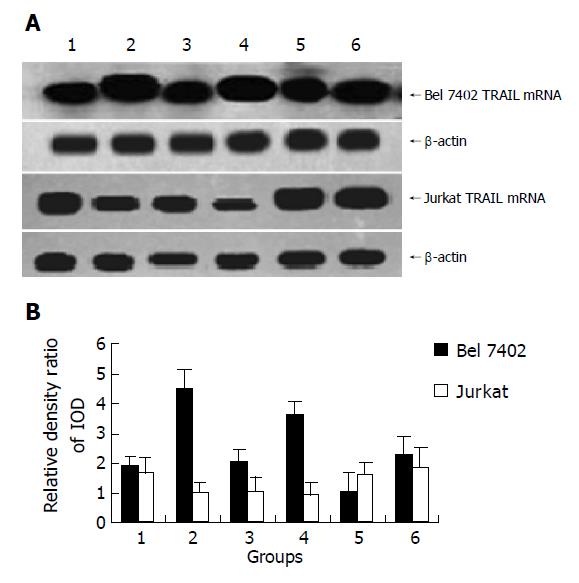
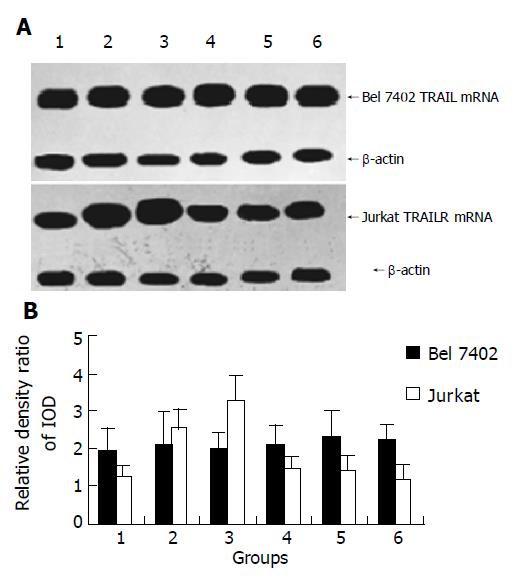
After being treated with AFP (20 mg/L), anti-AFP (40 mg/L), AFP (20 mg/L) plus anti-AFP (40 mg/L) for 12 h, it showed that AFP could enhanced the expression of TRAIL mRNA apparently, but no significant influence on the expression of TRAILR mRNA was detected in Bel7402 cells. The role of AFP in Jurkat cells indicated that APF could inhibit the expression of TRAIL mRNA and promote the expression of TRAILR mRNA. When Bel7402 cells and Jurkat cells were co-incubated, AFP functioned co-operatively with Bel 7402 cells to restrain the expression of TRAIL mRNA in Jurkat cells, AFP could not synergize with Bel 7402 cells to stimulate the expression of TRAILR mRNA in Jurkat cells. Anti-AFP had no significant influence on the expression of TRAIL mRNA and TRAILR mRNA in the two types of cells, but it could prohibit the function of AFP (Figures 3 and 4).
Figure 3 Effect of AFP (20 mg/L), anti-AFP (40 mg/L) and AFP (20 mg/L) plus anti-AFP (40 mg/L) on the TRAIL mRNA expression of Bel7402 cells and Jurkat cells were cultured individually or cultured together.
A: Northern blot of TRAIL mRNA of Bel7402 cells and Jurkat cells; B: Quantitative analysis IOD of TRAIL/IOD of β-actin, the columns represent mean±SD.
Figure 4 Influence of AFP (20 mg/L), anti-AFP (40 mg/L) and AFP (20 mg/L) plus anti-AFP (40 mg/L) on the expression of TRAILR mRNA in Bel7402 cells and Jurkat cells were cultured individually or cultured together.
A: Northern blot analysis of TRAILR mRNA of Bel7402 cells and Jurkat cells; B: Quantitative analysis IOD of TRAILR/IOD of β-actin, the columns represent mean±SD.
Figure 3, lane 1: Control groups (cells were cultured individually, respectively); lane 2: AFP treated groups (cells were cultured individually, respectively); lane 3: Cells were co-cultured groups; lane 4: Cells were co-cultured and treated with AFP group; lane 5: Cells were co-cultured and treated with anti-AFP group; lane 6: Cells were co-cultured and treated with AFP plus anti-AFP group.
Figure 4, Lane 1: Control groups (cells were cultured individually, respectively); lane 2: AFP treated groups (cells were cultured individually, respectively); lane 3: Cells were co-cultured groups; lane 4: Cells were co-cultured and treated with AFP group; lane 5: Cells were co-cultured and treated with anti-AFP group; lane 6: Cells were co-cultured and treated with AFP plus anti-AFP group.
DISCUSSION
It has been well known that AFP plays a role in inhibiting immunity rejection and cellular immune response during embryo development[16,17]. The synthesis and secretion of AFP always accompany liver cell growth. Researches have proved that AFP could promote the proliferation of various tumor cells, such as liver cancer cells[3,6]. For tumor cell growth in vivo, one of the mechanisms is to escape from immune surveillance. Lymphocytes play a very important role in inhibiting the proliferation of tumor cells. Previous investigations have confirmed the inhibitory effect of AFP on lymphocyte responses of cancer patients[18-20].
It has been known that escape of AFP from the immunity monitors may lead to the proliferation and survival of cancer cells. Researches have documented that AFP has a capability of regulating the growth of various cells. However, the mechanism of AFP in controlling the growth and immune escape of liver cancer cells is unclear. Formerly, it was thought that AFP could carry unsaturated fatty acids, which are essential nutrients for cell growth, AFP in this manner may promote the metabolism of unsaturated fatty acids to yield inositol triphosphate (IP3) which can affect the signal transduction of cells[21]. As a result, liver cancer cells are maintained to grow in vivo. The present research showed that AFP could promote the expression of FasL and TRAIL, and suppress the expression of Fas in hepatoma cells, but the expression of TRAILR was not significantly influenced, and an opposite role of AFP in Jurkat lymphocytes was indicated. The fact that AFP could inhibit the expression of TRAIL and FasL, and promote the expression of Fas and TRAILR when Jurkat cells and Bel 7402 cells were co-incubated, the results also indicated AFP synergism with Bel7402 cells to restrain the expression of FasL and TRAIL in Jurkat cells. FasL and Fas are a pair of systems that can induce cell death (apoptosis). Fas is defined as the death receptor and may induce the apoptotic process of cells by combining with its natural ligand FasL[22-25]. There is evidence that Bcl-2 protein displays a reciprocal pattern of expression during the development of lymphocytes. Bcl-2 family is a participant in the pathway that leads to cell apoptosis by mediating the signals from “death receptors” known as Fas on the cell surface, and Bcl-2 could affect the apoptosis of hepatocellular cancer cells through Fas[26,27], but this apoptosis-inducing effect has non-specificity of cells. Tumor cells can escape from the attack of lymphocytes by changing its Fas/FasL system[28,29]. TRAIL is mainly expressed in activated lymphocytes and can combine specifically with its receptor to induce apoptosis of tumor cells, but not for normal cells[30-33]. Lots of researches have found that the FasL/Fas system plays a very important role in promoting apoptosis and escaping from host immune surveillance of hepatocellular cancer cells[34-38]. The high expression of Fas in hepatocellular cancer cells can be beneficial to the growth of tumor cells[37,39]. Hepatocellular cancer cells could suppress the infiltrated lymphocytes to express FasL, which triggers hepatoma cells to escape from the immunity attack of lymphocytes[12,40,41]. The present results showed that AFP not only reduced Fas expression in cancer cells, which could suppress the attack of lymphocytes, but also promoted the expression of FasL in cancer cells and the expression of Fas in lymphocytes. It may be speculated that FasL of tumor cells may induce apoptosis of lymphocytes by combining its Fas. In the present study, FasL of Bel7402 cells could bind to Fas of Jurkat cells that lead the lymphocytes to apoptosis. This may be one of the mechanisms of hepatocellular cancer cells escaping from host immune surveillance.
The reason why TRAIL could induce tumor cell apoptosis is complicated. It has been reported that TRAIL may induce cell apoptosis through its five kinds of receptors[42-44]. Recently, it has been found that the effect of TRAIL was mediated through death receptors DR4 and DR5 on the membrane of tumor cells, which could combine with TRAIL to trigger caspase signal transduction pathways and induce tumor cell apoptosis[45]. Some studies have verified that TRAIL gene transferring adenoviral vector system could inhibit proliferation of human hepatoma SMMC7721 cells and induce cell apoptosis, TRAIL has been considered as an anticancer cytokine, which may generate a novel strategy for the treatment of hepatocellular carcinoma. Our data showed that AFP could enhance the expression of TRAIL, and had no obvious influence on the expression of DR4 in Bel7402 cells. On the other hand, the effect of AFP in Jurkat cells showed that it could promote the expression of DR4 and suppress the expression of TRAIL. The co-operative effect of the level of DR4 elevated by AFP in Jurkat cells and elevated TRAIL in Bel 7402 cells could facilitate apoptosis of infiltrated lymphocytes. Thus, AFP secreted from liver cancer cells could play a role in protecting itself in escaping from immune attack during the growth of liver cancer cells. In our previous studies, AFP could enhance tumor cell growth and the expression of some oncogenes[46-49]. The present study manifested that liver cancer cells persisted malignant proliferation in vivo, and secreted AFP which played a role not only in promoting cancer cells to cleavage, but also in triggering hepatoma cells to escape from host immune surveillance by altering the expression of TRAIL/TRAILR and Fas/FasL in hepatocellular cancer cells or in Jurkat lymphocytes.
Some researches showed that AFP was not necessary for the embryo development, but only required for female fertility[50]. A recent study revealed that AFP’s DNA vaccine had a distinctive antitumor immunopreventive effect on AFP-producing tumors. Some studies and our previous researches[46-49] have discovered that AFP could promote cancer cell growth by influenced cAMP and Ca2+ mediated signal transduction pathways[3,5,46-49]. AFP could cause apoptosis of cancer cells by activating caspase-3-like proteases rather than Fas[52]. These results showed that AFP had a complex biological function. It indicated that AFP could regulate the growth of tumor cells and lymphocytes by various intracellular signal pathways and at multiple regulation levels[17,52,53]. In this study, HSA as a negative control and mono-clonal antibody against AFP (anti-AFP) could not notably influence the expression of TRAIL/TRAILR and Fas/FasL in Bel7402 cells or in Jurkat cells. However, the fact that anti-AFP could block the function of AFP these indicates the high specificity of AFP in regulating the expression of TRAIL/TRAILR and Fas/FasL in the two types of cells. The present study showed that AFP could affect the expression of apoptosis related-genes of lymphocytes and hepatoma cells to maintain cancer cell growth in vivo. The precise mechanism of AFP contributes to a better understanding of the physiological effect of AFP on regulating tumor cell growth and escaping from host immune surveillance of liver cancer cells.