Published online Apr 28, 2005. doi: 10.3748/wjg.v11.i16.2526
Revised: May 28, 2004
Accepted: June 28, 2004
Published online: April 28, 2005
AIM: To evaluate the therapeutic efficacy of systemic chemo-immunotherapy for advanced hepatocellular carcinoma (HCC).
METHODS: Twenty-six patients with advanced HCC were treated by using systemic chemo-immunotherapy (PIAF regimen), which consisted of cisplatin (20 mg/m2) intravenously daily for 4 consecutive day, doxorubicin (40 mg/m2) intravenously on day 1, 5-fluorouracil (400 mg/m2) intravenously daily for 4 consecutive day, and human recombinant α-interferon-2a (5 Mu/m2) subcutaneous injection daily for 4 consecutive day. The treatment was repeated every 3 wk, with a maximum of six cycles.
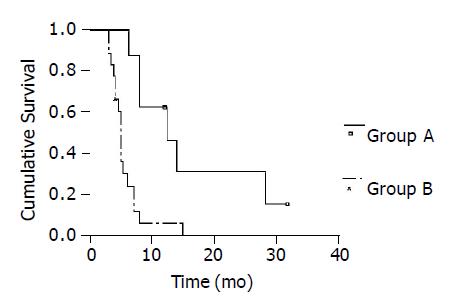
RESULTS: A total of 90 cycles of PIAF treatment were administered, with a mean number of 3.9 cycles per patient. Eight patients received six cycles of treatment (group A), and the remaining 18 were subjected to two to five cycles (group B). There were 0 complete response, 4 partial responses, 9 static diseases and 13 progressive diseases, with a disease control rate of 50% (13/26). The 1-year survival rate was 24.3%, with a median survival time of 6.0 mo. Group A had a remarkably better survival as compared with group B, the 1- and 2-year survival rates were 62.5% vs 6.1% and 32.3% vs 0%, and a median survival time was 12.5 mo vs 5.0 mo (P = 0.001).
CONCLUSION: Systemic chemo-immunotherapy using PIAF regimen represented an effective treatment and could improve the survival rate and prolong the survival time in selected patients with advanced HCC.
- Citation: Yin XY, Lü MD, Liang LJ, Lai JM, Li DM, Kuang M. Systemic chemo-immunotherapy for advanced-stage hepatocellular carcinoma. World J Gastroenterol 2005; 11(16): 2526-2529
- URL: https://www.wjgnet.com/1007-9327/full/v11/i16/2526.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i16.2526
Hepatocellular carcinoma (HCC) is one of the leading causes of death from malignancies worldwide[1]. Surgical resection offers the best hope of long-term survival[2,3]. However, only 9-37% of patients are candidates for surgical intervention due to advanced stage of the disease and/or impaired hepatic functional reserve[4,6].
Unresectable HCCs have been commonly treated by transcatheter arterial chemoembolization (TACE)[7-10] or image-guided local ablative therapies, including ethanol injection, radiofrequency ablation and microwave ablation[11-13]. However, their management, especially those with portal tumor thrombi or distant metastasis, still remains a major challenge. Systemic chemotherapy has been used as an alternative modality in clinical settings, unfortunately, its therapeutic efficacy appears disappointed[14].
Alpha-interferon (α-INF) is one kind of pleiotropic cytokines secreted by dendritic cells, macrophages and other hematopoietic cells in response to viral/bacterial infection[15]. In addition to its antiviral capacity, it has an anti-proliferative effect and a direct anti-tumoral activity[16-20]. Furthermore, it can enhance anti-tumoral effects of 5-fluorouracil (5-FU)[21-23]. Recently, a combined systemic chemo-immunotherapy consisting of α-INF, 5-FU, doxorubicin and cisplatin (known as PIAF regimen) was jointly designed by Chinese University of Hong Kong and the M.D. Anderson Cancer Center of United States, and has been shown to yield some remarkable outcomes for inoperable HCC[24,25].
In the present study, we evaluated the therapeutic efficacy of systemic chemo-immunotherapy for advanced-stage HCC.
A total of 26 patients with advanced-stage HCC were treated by using systemic chemo-immunotherapy (PIAF regimen) at our hospital between June 2000 and July 2003. The entry criteria included: age between 18 and 70 years, absence of cardiovascular and renal diseases, unresectability of tumors, good general status with Karnofsky performance score >80%, serum total bilirubin <51.3 μmol/L and absence of massive or intractable ascites, endogenous creatinine clearance rate >50 mL/min, white blood cell count >3×109/L and platelet count >100×109/L. Informed consent for treatment was obtained from each patient.
There were 23 males and 3 females, with a mean age of 46±10 years (range, 27 and 66 years). Twelve of them (46%) were primarily-treated cases, and the remaining 14 patients (54%) were recurrent cases after liver resection, with a time interval from initial hepatectomy of less than 1 year in 12 and more than 1 year in 2. Twenty-four of them (92.3%) were sero-positive for HBsAg. In pretreatment liver function status, 21 patients (80.8%) had grade A and 5 (19.2%) had grade B in Child-Pugh classification. Eighteen of them (69.2%) had an elevated serum alpha-fetoprotein (AFP) level of over 500 μg/L prior to treatment, with an average level of 32104±18819 μg/L (range, 1000 and 62273 μg/L).
Twenty-three out of 26 patients had intrahepatic tumors, including nodular type in 14, massive type in 5 and diffuse type in 4, with a mean dimension of 8.1±5.3 cm (range, between 1.0 and 16.4 cm). Portal trunk/major branch tumor thrombi were presented in 12 patients, superior mesenteric venous tumor thrombi in 1 and inferior vena cava tumor thrombi in 1. Nine patients had lung metastatic lesions, two had bone metastasis and one adrenal gland metastasis. According to UICC staging system, 1 patient had stage III, 16 had stage IVa and 9 stage IVb.
The PIAF regimen was illustrated in Table 1. One cycle of the treatment lasted for 4 d. Prior to initiation of the treatment, a bolus of 3 mg granisetron (Kytril) was given intravenously to relieve the adverse effects of nausea and vomiting. Non-steroid anti-inflammatory analgesics were used to relieve the fever caused by administration of α-INF-2a. The treatment was repeated at an interval of 3 wk, with a maximum number of six cycles. If the patients had white blood cell count <3×109/L prior to any cycle of treatment, granulocyte-macrophage colony-stimulating factor was administered and treatment was temporarily delayed until white blood cell count >3×109/L.
Complete blood cell count, liver function test and renal function test were carried out before and after each cycle of treatment. Any side effect that occurred during the treatment was recorded.
Before treatment, ultrasound investigation, computerized tomography scanning and chest X-ray examination were done to evaluate the size and extent of tumors. These imaging studies were repeated once after each of two to three cycles. In patients with pretreatment elevated AFP level, serum AFP was titrated before and after each cycle of treatment.
The therapeutic efficacy was evaluated as follows. Complete response (CR) was defined as complete disappearance of all known tumor nodules on imaging studies and normalization of serum AFP level for at least 4 wk. Partial response (PR) was defined as no less than 50% reduction in the size of the largest tumor nodule for at least 4 wk without presence of new lesions or progression of lesions. Static disease (SD) was defined as less than 50% reduction, or no more than 25% increase, in the size of the largest tumor nodule for at least 4 wk. Progressive disease (PD) was defined as more than 25% increase in the size of the largest tumor nodule for at least 4 wk or presence of new lesions.
The results were expressed as mean±SD. Statistical analysis was performed by using commercially-available SPSS software package version 10.0. Student’s t test and χ2 test were used to compare the differences of nominal and numerical variables between groups, respectively. Survival rate was calculated by using Kaplan-Meier method and log-rank method was used to compare its difference between groups. A two-tailed P<0.05 was considered statistically significant.
A total of 90 cycles of PIAF treatment were administered in 26 patients, with a mean number of 3.9±1.6 cycles per patient. Eight patients completed six cycles of treatment (group A), and the remaining 18 received two to five cycles (group B). The reasons why the patients were treated with less than six cycles included tumor progression in nine, intolerance to the treatment in six, death from massive upper gastrointestinal tract bleeding in two and death from heart disease in one. There were no significant differences in clini-copathological features between groups A and B (Table 2).
| Group A (n = 8) | Group B (n = 18) | |
| Gender (male:female) | 7:1 | 16:2 |
| Age (yr) | 46±9 | 45±10 |
| HBsAg (positive:negative) | 7:1 | 17:1 |
| Liver function (grade A:B) | 7:1 | 14:4 |
| AFP (>500 mg/L:<500 mg/L) | 6:2 | 12:6 |
| Primarily-treated: Recurrent | 2:6 | 10:8 |
| Tumor staging (III:IVa:IVb) | 0:5:3 | 1:11:6 |
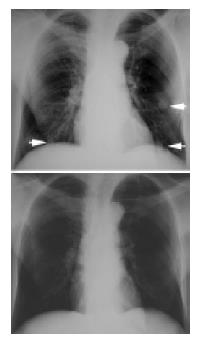
No CR was achieved in 26 patients. There were 4 PR (Figure 1), 9 SD and 13 PD. Both PR and SD indicated that the disease was brought under control, and the disease control rate was 50% (13/26).
In adverse effects, bone marrow suppression was the most common, including leukopenia in 14 patients, thrombocytopenia in 4 and erythrocytopenia in 2. The other side effects consisted of vomiting in 11, alopecia in 11, drug fever in 4, renal insufficiency in 2 and diarrhea in 2.
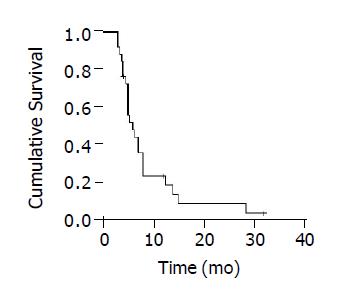
The mean follow-up time was 8.4±7.2 mo (range, 3 and 32.0 mo). Twenty-three patients were dead during follow-up. The death causes included tumor progression and/or liver function failure in 20, massive upper gastrointestinal bleeding in 2 and heart disease in 1. The remaining three were still alive. The 1-year survival rate was 24.3%, with a median survival time of 6.0 mo (Figure 2). Group A had a remarkably better survival in comparison with group B. The 1- and 2-year survival rates were 62.5% and 32.3% vs 6.1% and 0%, the median survival time was 12.5 mo vs 5.0 mo (log rank = 10.73, P = 0.001, Figure 3).
The management of advanced-stage HCC still remains a major challenge. Though TACE has been extensively used, there still exist some controversies about its therapeutic efficacy. Of the seven published randomized control trials comparing TACE with symptomatic treatment for advanced HCC, two showed a remarkable 2-year survival benefit of TACE, two indicated a likely trend, and the remaining three revealed no survival benefit of TACE[26]. Other image-guide percutaneous therapeutic modalities, such as ethanol injection, microwave ablation and radiofrequency ablation, were basically unsuitable for advanced diseases, especially for those with portal tumor thrombi and/or distant metastasis.
Systemic chemotherapy has been used as an alternative approach for advanced HCC, but it has been usually associated with a poor prognosis. A meta-analysis revealed that systemic monotherapy using adriamycin or 5-FU did not yield any survival benefit for patients with unresectable HCC[14].
Human recombinant α-INF is an immuno-modulatory cytokine. It has been widely used as an immunotherapeutic agent against various malignancies, including malignant melanoma[17], lymphoid malignancies[18], metastatic endocrine tumors[19], renal cell carcinoma[20,27] and leukemia[28] in clinical settings. Recently, Kaneko et al[16], demonstrated that α-INF could inhibit matrix metalloproteinase activity, thus yielding remarkable anti-proliferative and anti-metastatic effects on human liver cancer cells. PIAF chemo-immunotherapy represents one kind of combined regimens. It consists of three chemotherapeutic agents with different anti-tumoral mechanisms, thus helping enhance the tumoricidal efficacy and minimize the adverse effects. Moreover, the regimen takes advantages of synergistic anti-tumoral effects between α-INF and 5-FU. Recently, Leung et al[25], employed PIAF regimen to treat a series of 149 patients with unresectable HCC, 18.1% of them were in UICC stage I or II, 14.8% in stage III and 69.1% in stage IV. A PR rate of 14.8% and a median survival time of 7.2 mo were reported.
In the present series, the vast majority of patients had a late-stage disease, with presence of portal trunk tumor thrombi and/or extrahepatic metastasis in over 80%. According to UICC classification, over 96% of them were in stage IV. Such a late-staged disease was usually associated with a median survival time of no more than 3-4 mo. PIAF treatment brought the disease under control in 50% of patients, and yielded a 1-year survival rate of 24.3%, with a median survival time of 6.0 mo. Furthermore, the patients who completed six therapeutic cycles had a much better prognosis. The 1- and 2-year survival rates reached up to 62.5% and 32.3%, respectively, with a median survival time of 12.5 mo. In terms of therapeutic toxicity, bone marrow suppression took place at the greatest frequency, but was usually mild and transient. The treatment seemed to be well tolerant in most patients. Our results clearly indicate that PIAF treatment represents an effective treatment, and can improve the survival rate and prolong the survival time in selected patients with advanced HCC.
The authors thank Dr. Z. Sheng Guo, an assistant professor from Division of Surgical Oncology, University of Pittsburgh Cancer Institute, USA, for his critical reading of the manuscript.
| 1. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3280] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 2. | Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, Wong J. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 467] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 3. | Ercolani G, Grazi GL, Ravaioli M, Del Gaudio M, Gardini A, Cescon M, Varotti G, Cetta F, Cavallari A. Liver resection for hepatocellular carcinoma on cirrhosis: univariate and multivariate analysis of risk factors for intrahepatic recurrence. Ann Surg. 2003;237:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 262] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 4. | Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg. 2002;235:466-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 308] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 5. | Lau WY, Leung TW, Yu SC, Ho SK. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg. 2003;237:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 179] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Yu AS, Keeffe EB. Management of hepatocellular carcinoma. Rev Gastroenterol Disord. 2003;3:8-24. [PubMed] |
| 7. | Vogl TJ, Trapp M, Schroeder H, Mack M, Schuster A, Schmitt J, Neuhaus P, Felix R. Transarterial chemoembolization for hepatocellular carcinoma: volumetric and morphologic CT criteria for assessment of prognosis and therapeutic success-results from a liver transplantation center. Radiology. 2000;214:349-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | O'Suilleabhain CB, Poon RT, Yong JL, Ooi GC, Tso WK, Fan ST. Factors predictive of 5-year survival after transarterial chemoembolization for inoperable hepatocellular carcinoma. Br J Surg. 2003;90:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2605] [Article Influence: 113.3] [Reference Citation Analysis (0)] |
| 10. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1985] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 11. | Iannitti DA, Dupuy DE, Mayo-Smith WW, Murphy B. Hepatic radiofrequency ablation. Arch Surg. 2002;137:422-426; discussion 427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Lu MD, Chen JW, Xie XY, Liu L, Huang XQ, Liang LJ, Huang JF. Hepatocellular carcinoma: US-guided percutaneous microwave coagulation therapy. Radiology. 2001;221:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 137] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Komorizono Y, Oketani M, Sako K, Yamasaki N, Shibatou T, Maeda M, Kohara K, Shigenobu S, Ishibashi K, Arima T. Risk factors for local recurrence of small hepatocellular carcinoma tumors after a single session, single application of percutaneous radiofrequency ablation. Cancer. 2003;97:1253-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 253] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 14. | Treiber G. Systemic treatment of hepatocellular carcinoma. Dig Dis. 2001;19:311-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Hertzog PJ, O'Neill LA, Hamilton JA. The interferon in TLR signaling: more than just antiviral. Trends Immunol. 2003;24:534-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Kaneko F, Saito H, Saito Y, Wakabayashi K, Nakamoto N, Tada S, Suzuki H, Tsunematsu S, Kumagai N, Ishii H. Down-regulation of matrix-invasive potential of human liver cancer cells by type I interferon and a histone deacetylase inhibitor sodium butyrate. Int J Oncol. 2004;24:837-845. [PubMed] |
| 17. | Hancock BW, Harris S, Wheatley K, Gore M. Adjuvant interferon-alpha in malignant melanoma: current status. Cancer Treat Rev. 2000;26:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Tsimberidou AM, Giles F, Romaguera J, Duvic M, Kurzrock R. Activity of interferon-alpha and isotretinoin in patients with advanced, refractory lymphoid malignancies. Cancer. 2004;100:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Faiss S, Pape UF, Böhmig M, Dörffel Y, Mansmann U, Golder W, Riecken EO, Wiedenmann B. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors--the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21:2689-2696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 20. | Donskov F, Marcussen N, Hokland M, Fisker R, Madsen HH, von der Maase H. In vivo assessment of the antiproliferative properties of interferon-alpha during immunotherapy: Ki-67 (MIB-1) in patients with metastatic renal cell carcinoma. Br J Cancer. 2004;90:626-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Choi EA, Lei H, Maron DJ, Mick R, Barsoum J, Yu QC, Fraker DL, Wilson JM, Spitz FR. Combined 5-fluorouracil/systemic interferon-beta gene therapy results in long-term survival in mice with established colorectal liver metastases. Clin Cancer Res. 2004;10:1535-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3255] [Cited by in RCA: 3609] [Article Influence: 164.0] [Reference Citation Analysis (0)] |
| 23. | Mitchell MS. Combinations of anticancer drugs and immunotherapy. Cancer Immunol Immunother. 2003;52:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Lau WY, Leung TW, Lai BS, Liew CT, Ho SK, Yu SC, Tang AM. Preoperative systemic chemoimmunotherapy and sequential resection for unresectable hepatocellular carcinoma. Ann Surg. 2001;233:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Leung TW, Tang AM, Zee B, Yu SC, Lai PB, Lau WY, Johnson PJ. Factors predicting response and survival in 149 patients with unresectable hepatocellular carcinoma treated by combination cisplatin, interferon-alpha, doxorubicin and 5-fluorouracil chemotherapy. Cancer. 2002;94:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2267] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 27. | Atzpodien J, Royston P, Wandert T, Reitz M. Metastatic renal carcinoma comprehensive prognostic system. Br J Cancer. 2003;88:348-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 197] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Delannoy A, Cazin B, Thomas X, Bouabdallah R, Boiron JM, Huguet F, Straetmans N, Zérazhi H, Vernant JP, Dombret H. Treatment of acute lymphoblastic leukemia in the elderly: an evaluation of interferon alpha given as a single agent after complete remission. Leuk Lymphoma. 2002;43:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |