Published online Apr 28, 2005. doi: 10.3748/wjg.v11.i16.2444
Revised: February 28, 2004
Accepted: June 7, 2004
Published online: April 28, 2005
AIM: To make drug sera of Salvia miltiorrhiza and Yigankang, both of which are Chinese herbs that activate bleeding and eliminate stasis, in normal rats and those with liver fibrosis, respectively. To investigate and compare the effects of the two different drug sera on the proliferation and activation of hepatic stellate cells (HSCs).
METHODS: Some rats were induced with liver fibrosis: 40% carbon tetrachloride (CCl4) subcutaneous injection, twice a week for 9 wk. Salvia miltiorrhiza, Yigankang, colchicines and normal saline were administered into the stomachs of normal rats and those with liver fibrosis. Drug sera were extracted 5 d later. HSCs in vitro were cultivated in different drug sera for 24 h. The rates of proliferation and expression of α-smooth muscle actin (α-SMA) were detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and immunocyt-ochemistry stain, respectively.
RESULTS: The drug sera from normal and liver fibrotic rats could be used to cultivate HSCs and to observe the effects of the corresponding components of herbs on HSCs. Salvia miltiorrhiza and Yigankang had better inhibitory effects on HSCs than colchicines (MTT: normal drug serum: Salvia miltiorrhiza 0.42±0.08, Yigankang 0.32±0.10 vs colchicines 0.45±0.12 pathological drug serum: Salvia miltiorrhiza 0.33±0.02, Yigankang 0.26±0.01 vs colchicines 0.41±0.09. P<0.05). The drug sera of Salvia miltiorrhiza, Yigankang from liver fibrotic rats had a stronger inhibitory effect than the same ones from normal rats (MTT: Salvia miltiorrhiza: normal drug serum 0.42±0.08 vs pathological drug serum 0.33±0.02. Yigankang: normal drug serum 0.32±0.10 vs pathological drug serum 0.26±0.01. P<0.05).
CONCLUSION: Salvia miltiorrhiza and Yigankang could inhibit the expression of α-SMA and the proliferation of HSCs. The drug sera from normal and liver fibrotic rats had different effects on HSCs, probably due to different metabolic processes, effective components and different quantities of drug contents in drug sera from rats with different states of liver.
- Citation: Yao XX, Lv T. Effects of pharmacological serum from normal and liver fibrotic rats on HSCs. World J Gastroenterol 2005; 11(16): 2444-2449
- URL: https://www.wjgnet.com/1007-9327/full/v11/i16/2444.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i16.2444
The activation and proliferation of hepatic stellate cells (HSCs) is the major cellular basis for the formation of liver fibrosis[1,2]. No ideal Western medicine has been developed against liver fibrosis until now, otherwise, a series of blood-activating and stasis-eliminating Chinese medicine, such as Salvia miltiorrhiza, has a positive effect on the preventive treatment and inversion of liver fibrosis[3,4]. But it is difficult to clarify the working mechanism and real effective components of such Chinese medicine against fibrosis. A feasible way to solve this problem appeared when the seropharmacological method was advanced in 1984[5]. By traditional seropharmacological method, the drug sera are always extracted from normal rats which have been induced with drugs[6]. But it is the patients with hepatic diseases who take the corresponding drugs clinically. What is the difference between normal and pathologic livers during the metabolic process? The project has been carried out to find out if there exist differences in the working effects and effective components of the same drugs caused by different states of liver.
HSC cell line: CFSC is established and presented by Professor Greenwell, Marion Bessin Liver Research Center, Albert Einstein College of Medicine. The phenotype of CFSC is HSC which has been activated. They were obtained from CCl4-cirrhotic liver of rats, after spontaneous immortalization in culture.
Eighty of clean, male Sprague-Dawley (SD) rats of 200-250 g were from the Laboratory Animal Center of Hebei Medical University. RPMI-1640 culture medium, L-Glutamine, hydroxyethyl piperazine ethanesulfonic acid (HEPES), pancreatin, MTT, dimethyl sulfoxide (DMSO), antibody of α-SMA were all from Biological Technological Company. Carbon tetrachloride (CCl4) and other materials were all analytically pure (AP). Chinese herbs were from Lerentang Chinese Medicine Drugstore.
SD rats 80: 200-250 g, male, were randomized into eight groups, 10 in each group.
Group A: normal rats.
A1: Salvia miltiorrhiza A2: Yigankang A3: colchicines A4: normal control (normal saline).
Group B: liver fibrotic rats.
B1: Salvia miltiorrhiza B2: Yigankang B3: colchicine B4: fibrosis control (normal saline).
The rats in group A were all normal and healthy. The rats in groups A1-A4 were fed with corresponding drugs or normal saline. The rats in group B were all induced with liver fibrosis, then corresponding drugs or normal saline were fed.
The rats in group B were induced with liver fibrosis by 40% CCl4, subcutaneous injection, 4 mL/kg the first time, then 2 mL/kg, twice a week, for 9 wk.
Salvia miltiorrhiza and Yigankang were from Lerentang Drugstore of Hebei Province. They were decocted and concentrated. The solutions were sealed and kept in 4 °C. The quantities of raw herbs in solution: Salvia miltiorrhiza 0.51 g/mL, Yigankang 0.72 g/mL. Colchicines (tablets) were dissolved in normal saline to form 0.04 mg/mL.
Corresponding drugs were poured into the stomachs of rats for 5 d. The quantity of drugs is 10 times that of the normal adults per kilogram per day, twice a day. Rats were fasted since the night of the 4th day. Blood was extracted from inferior vena cava 2 h after the drugs were given on the fifth morning. Then serum was obtained by centrifugation, 3000 r/min, 4 °C, for 20 min. The serum from the rats in the same group were mixed, then were inactivated at 56 °C, for 30 min. The sera were stored at -70 °C. Drug sera sterilized by filtration, were dissolved in 8% new calf serum (NCS)/RPMI-1640 cell culture medium to produce 10% drug serum-1640 culture medium, which was ready for use at -20 °C.
Cell solutions were incubated on 96-well plate. When HSCs grew to 90%, they were cultivated in pure RPMI-1640 (no serum) overnight, so as to synchronize HSCs into the G0 period. The next morning, corresponding groups of 10% drug serum-1640 media in the wells were changed. There were nine such holes for each kind of drug serum. The results were obtained from an average of nine numbers. A group of control was set at the same time. Twenty microliters of MTT was added after the drug serum was allowed to have worked for 24 h. Then DMSO was added 4 h later. Optical density (A) in all holes was measured. Then the inhibitory rate (IR) of all drug sera on the growth of HSCs were calculated. IR = (Aexperiment group-Acontrol group)/Acontrol group×100%.
HSCs were transferred onto the bottle chip. HSCs were cultured in pure RPMI-1640 overnight when HSCs grew to 90% on the chip. There were three similar chips for each kind of drug serum. The chips were taken out after being cultivated in drug serum for 24 h. HSCs were fixated. Antibody of α-SMA was added on HSCs, then SP immunocytochemistry stain was performed. The rate of positive cells was obtained by analyzing the stain (using the analysis software from Huadong Normal University) under microscope (×200). Final results were obtained from the average.
SPSS 10.0 was used to perform F and χ2 test and P<0.05 was regarded as statistically significant.
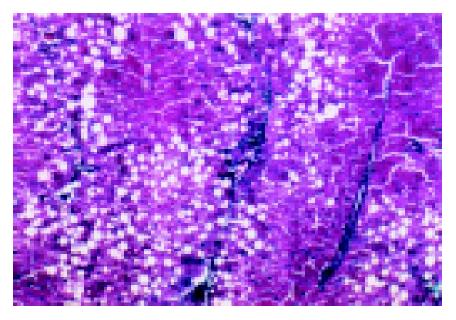
Obvious proliferation of fibers appeared in the liver when the rats in group B were modeled for 9 wk. There were inflammation necrosis and vacuole degeneration in the liver. No obvious changes appeared in the rat livers of group A. (Figure 1).
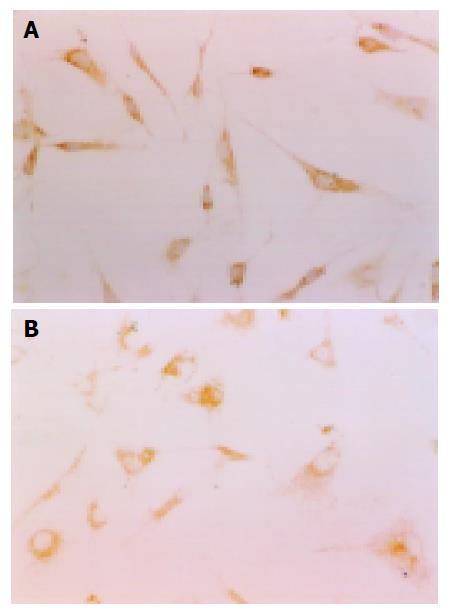
The experiment of pure sera from normal and fibrotic rats on HSCs in vitro for 48 h showed that the RPMI-1640 culture media with serum from normal and fibrotic rats could keep the growth of HSCs’ normal. The effect of drug sera from normal and fibrotic rats on HSCs for 48 h showed that drug sera from three kinds of drugs could be used to cultivate HSCs in vitro, but HSCs’ growth and differentiation were very slow. The typical phenotype of HSCs was disappearing gradually; synapses were getting fewer and smaller; and cell bodies expanded. All showed that components in drug sera had worked on HSCs (Figure 2).
The detection of IR of drug sera on HSCs growth by MTT showed that Salvia miltiorrhiza, Yigankang inhibited the proliferation of HSCs more than colchicines (P<0.05). The drug sera from rats in groups B1 and B2 (sera of Salvia miltiorrhiza, Yigankang from fibrotic rats) had a stronger effect than that from A1 and A2 (sera of Salvia miltiorrhiza, Yigankang from normal rats) (P<0.05) (Table 1).
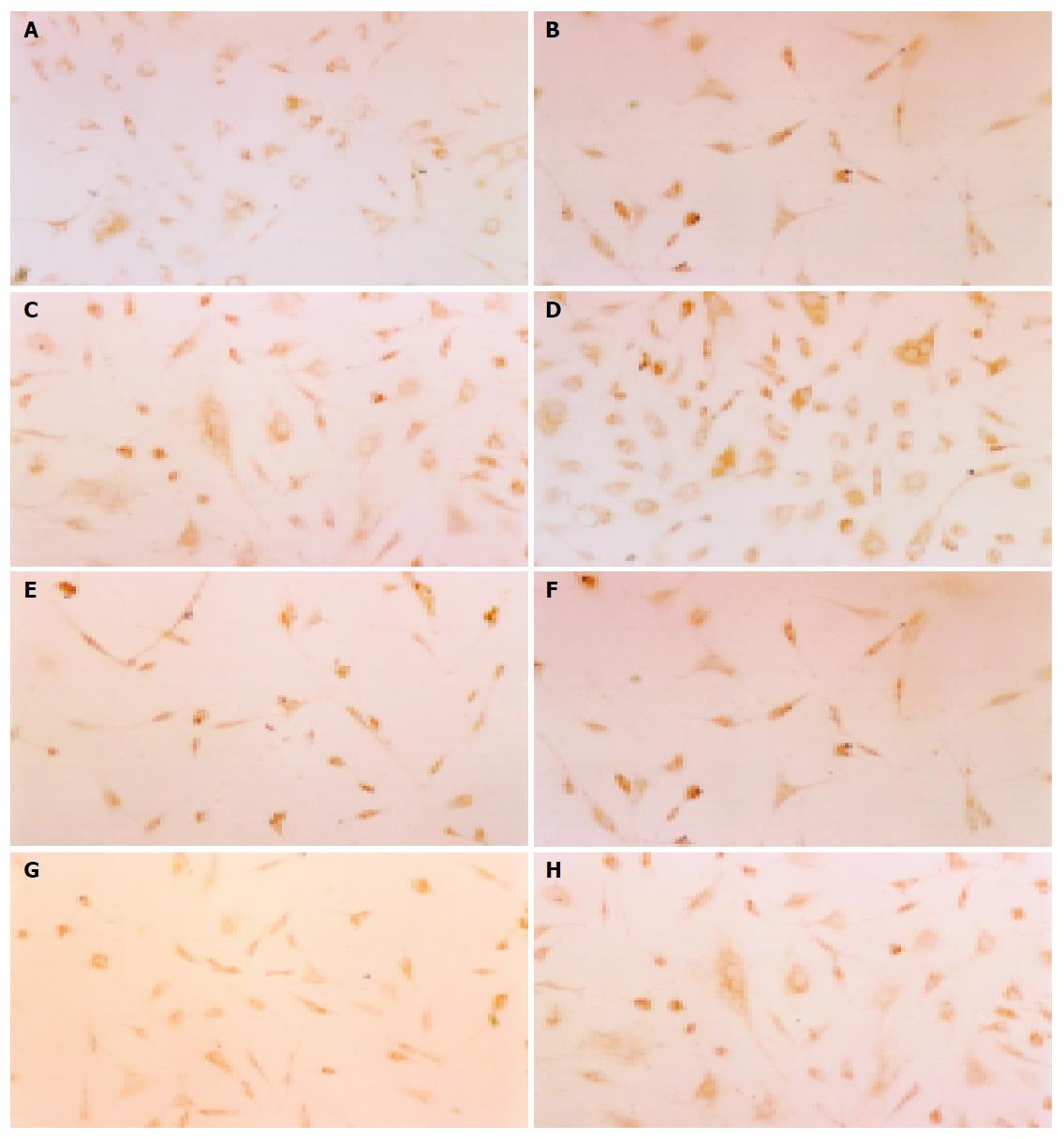
The detection of α-SMA in HSCs by SP immunocyt-ochemistry stain showed that the drug sera of Salvia miltiorrhiza and Yigankang from normal and fibrotic rats could all inhibit the expression of α-SMA, compared with colchicines (P<0.05). The drug sera from B1 and B2 had a stronger effect than that from A1 and A2 (P<0.05) (Figure 3, Table 2).
Today, chronic hepatic diseases caused by hepatitis virus, and the following liver fibrosis, cirrhosis and a few of liver cancer are still a serious problem, which is harmful to people’s health in China. Liver fibrosis is a necessary stage during the development of liver cirrhosis[7,8]. Therefore the preventive treatment and inversion of liver fibrosis is the key point to cure chronic hepatic diseases and to improve the prognosis. Now the researches mainly focus on how to inhibit and cut the initiative factor of fibrosis-the activation and proliferation of HSC[9]. No ideal Western drug and therapeutic method have emerged until now[10]. Otherwise, a few blood-activating and stasis-eliminating Chinese medicine, such as Salvia miltiorrhiza, Yigan Granule (Yigankang), show a positive effect in clinical and experimental studies[11,12]. Based on Yigan infusion, YigankangY is a new generation drug which was developed in recent years, and shows outstanding superiority in treating liver fibrosis (A recipe of Traditional Chinese Medicine, TCM, by Professor Xi-Xian Yao, who is a famous expert of gastrointestinal disease in China. The medicine has been commonly put into clinical use for 18 years, widely welcomed by sufferers because of its minimal side effects and excellent clinical efficiencies). But it is difficult to clarify the effective components of herbs which consist of complex parts. It is also a bottleneck that obstructs the development and application of new TCM. Chinese herbs come mostly from nature. They could be taken only after being dried and decocted under certain conditions. Also certain kinds of herbs do not have specific and exact structures like Western medicine, quite compressed to make complex prescriptions. Some nonpharmocological effect may occur if the herbs were added into the culture media of HSCs in vitro directly. Some drugs and drug precursors work through the effective substance which was produced after being transformed in vivo[13], or real effective components were physiologically active matters which were induced in vivo by the drugs. Such kind of drugs could be believed to be useless if they are added into the cell lines in vitro directly[14,15]. How to represent the effective components and the working mechanism of TCM by organic metabolism is the key problem.
The results of this research show that the drug sera of blood-activating and stasis-eliminating Chinese medicine such as Salvia miltiorrhiza, Yigankang (complex prescription) had an apparently inhibitory effect on HSC proliferation and the expression of α-SMA, which is the marker of the activation of HSCs[16,17]. The expressive rate of α-SMA on HSC of Salvia miltiorrhiza, Yigankang was 37.13%, 32.13%, respectively, which were lower than that of colchicines and normal saline (46.12%, 60.88%, respectively) obviously (P<0.05) (Table 2, Figure 3). The inhibitory rate of Salvia miltiorrhiza and Yigankang on HSC proliferation was 47.42%, 58.12%, respectively, which were higher than that of the control group (P<0.05) (Table 1). So it might be considered that some effective components were produced when Salvia miltiorrhiza, Yigankang was taken into the body and transformed after they were decocted. The effective components enter the liver and inhibit the activation and proliferation of HSC through certain mechanisms (such as inhibiting lipid peroxidation[18,19]). But the exact working components and their structures need further investigation.
In this experiment we mimicked the real condition of the usage of herbs clinically and in the manner of their disposal afterwards, the style of usage, the dosage and the metabolic process in vivo. The drug sera thus obtained contain the real working components which are produced after the metabolic process in vivo and then enter the serum. To research the effect of the sera on the function and the phenotype of HSCs, we applied the sera to cell culture in vitro in order to clarify the working mechanism of TCM. This is the traditional “pharmacological method”[20]. At the same time we noticed that, usually it is patients with liver diseases that take the herbs clinically. Different (normal and pathological) conditions of liver, which is the biggest organ for biological metabolism, could lead to some difference in the conversion of the same drug. The final effective components in the sera and the effects may differ when the same drug is metabolized by the body with different states of liver. In traditional pharmacological method, healthy rats are the drug receivers, and drug serum resources. So the aim of the study is to improve the traditional seropharmacological method in order to make the drug serum provider animals resemble the real body condition of clinical drug users. So we could mimic the possible condition, possible process, the products of the herbs’ metabolism in vivo, and patients’ condition clinically to the greatest extent, more exact than traditional “pharmacological method”. Thus we call it “modified seropharmacological method”.
The results of the research showed that, the drug sera of Salvia miltiorrhiza and Yigankang from liver fibrotic rats have a stronger inhibitory effect on the activation and proliferation of HSCs than the corresponding serum from normal rats. At the same time, the drug serum of Salvia miltiorrhiza from fibrotic rats inhibits the proliferation of HSCs at the rate of 47.42%, which is higher than the drug serum of Salvia miltiorrhiza from normal rats: 33.62% (P<0.05) (Table 1). The expressive rates of α-SMA on HSCs in the sera of Salvia miltiorrhiza from fibrotic and normal rats are 37.13%, 41.72% (P<0.05) (Table 2, Figure 3). The drug sera of Yigankang from fibrotic and normal rats inhibited the proliferation of HSCs at the rate of 58.12%, 49.74% (P<0.05) (Table 1). The rate of expression of α-SMA in HSCs in the sera of Yigankang from fibrotic and normal rats were 32.13%, 37.00%, respectively (P<0.05) (Table 2, Figure 3). So we considered that the drug sera extracted from liver fibrosis could reflect the metabolic process better, products and working components of the TCM against liver fibrosis in vivo theologically, and this kind of drug serum could be applied in the experimental study of TCM against liver fibrosis. The effects of pathological drug sera from liver fibrotic rats were strengthened than those from normal rats (both Salvia miltiorrhiza and Yigankang), which may be related with the different drug effects caused by different functional status of livers. From the Chinese traditional theory of “diagnosis based on different symptoms and pathogenesis” in Chinese traditional medicine[21], TCM will work best only if the symptoms and etiology are best suited to the indication. Both Salvia miltiorrhiza and Yigankang, are blood-activating and stasis-eliminating herbs; undoubtedly they indicate liver fibrosis. Normal rats had no illness or symptoms, so the effects of blood-activating and stasis-eliminating are not obvious. So the blood-activating and stasis-eliminating herbs such as Salvia miltiorrhiza and Yigankang might have a better effect on fibrotic rats. We considered that different functional status of livers metabolized drugs differently, so there was a difference in metabolic effective components and their content.
The drug sera that come from pathological liver (fibrotic model) (pathological drug serum) inhibited the activation and proliferation of HSCs more strongly than those from normal rats. The following factors may work. (1) TCM has an advantage to doubly regulate the physiological functions. They have no obvious effect on the normal liver cells, but perform a better regulatory effect on pathological liver, thereby making an effective indication. (2) In liver fibrosis models caused by CCl4, some responsive factors such as toxins and inhibitory cytokines induced by CCl4in vivo entered the serum, which cannot be inactivated by the pathological (fibrotic) liver may inhibit the HSC in vitro. The inhibitory effect of this kind of inhibitory cytokines may not be HSC specific, which is to say that, they could inhibit the activation and proliferation of many kinds of cells in vivo besides HSC. (3) The ability of the liver to transform and inactivate drugs decreases because the metabolic function of the fibrotic livers decreases generally. So it makes the concentration of the effective components higher, and prolong the working time, so as to inhibit HSCs more (Figure 3). Further researches needs to be performed to clarify the reason why drug sera from normal and fibrotic rats have different effects on HSC. For example, the rats could be modeled into liver fibrosis not by CCl4 in order to know whether or not the effect caused by nonspecific factors induced by CCl4 exists. Also we can optimize the conditions of serum inactivation so as to inactivate nonspecific factors. Or we can carry out the comparative study of pharmacokinetics after healthy volunteers and fibrotic patients take the same drugs, then compare the corresponding effective components in drug sera. Thus we can know the real reason why pathological drug sera work stronger, and modify the pharmocological method further.
Blood-activating and stasis-eliminating Chinese herbs such as Salvia miltiorrhiza and Yigankang have obvious effects on liver fibrosis[22,23]. But there is no ideal way to clarify the effective components among them. The value of this research lies in that we could get the real effective components of Salvia miltiorrhiza and Yigankang after they were metabolized by normal and fibrotic liver, which could provide reliable samples for analyzing the working components in the sera by HPLC, etc. Still there are difficulties in analyzing the sort, quantity and structure of effective components in the serum. It is also the key problem that needs to be solved in the study and development of new TCM. Making the drug sera of effective TCM against liver fibrosis by “modified seropharmacological methods”, by analyzing the effective part may be a feasible way to get the working part in the drug sera which are effective against fibrosis. It is also an important direction for future development. It might be possible to analyze the components and structures of the effective parts in drug sera against liver fibrosis, by high volume gene microarray chips, etc. It is also one of the important tasks in the research of TCM in future.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Weiskirchen R, Kneifel J, Weiskirchen S, van de Leur E, Kunz D, Gressner AM. Comparative evaluation of gene delivery devices in primary cultures of rat hepatic stellate cells and rat myofibroblasts. BMC Cell Biol. 2000;1:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Vincent KJ, Jones E, Arthur MJ, Smart DE, Trim J, Wright MC, Mann DA. Regulation of E-box DNA binding during in vivo and in vitro activation of rat and human hepatic stellate cells. Gut. 2001;49:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Zhang M, Song G, Minuk GY. Effects of hepatic stimulator substance, herbal medicine, selenium/vitamin E, and ciprofloxacin on cirrhosis in the rat. Gastroenterology. 1996;110:1150-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Nan JX, Park EJ, Kang HC, Park PH, Kim JY, Sohn DH. Anti-fibrotic effects of a hot-water extract from Salvia miltiorrhiza roots on liver fibrosis induced by biliary obstruction in rats. J Pharm Pharmacol. 2001;53:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Zhang QH, Zhong B, Chen KJ. Effect of concentrated xuefu zhuyu pill on proliferation of vascular smooth muscle cells in experimental atheriosclerosis rabbits observed by serologic pharmacological test. Zhongguo ZhongXiYi JieHe ZaZhi. 1996;16:156-159. [PubMed] |
| 6. | Xue DY, Hong JH, Xu LM. Salianic-acid B inhibits MAPK signaling in activated rat hepatic stellate cells. Zhonghua GanZangBing ZaZhi. 2004;12:471-474. [PubMed] |
| 7. | Wu J, Zern MA. Hepatic stellate cells: a target for the treatment of liver fibrosis. J Gastroenterol. 2000;35:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Lee KS, Buck M, Houglum K, Chojkier M. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest. 1995;96:2461-2468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 406] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 9. | Mühlbauer M, Bosserhoff AK, Hartmann A, Thasler WE, Weiss TS, Herfarth H, Lock G, Schölmerich J, Hellerbrand C. A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology. 2003;125:1085-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Shimizu I. Antifibrogenic therapies in chronic HCV infection. Curr Drug Targets Infect Disord. 2001;1:227-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Yao XX, Fu YL, Li XL. A multi-central study of effect of Yi杇an infusion on 324 cases of chronic hepatitis. Hebei Yixueyuan Xuebao. 1989;10:231-233. |
| 12. | Jiang SL, Li XT, Yao XX. Efficacy of profhylaxis and treatment of Yigankang on rat with hepatic fibrosis. Zhongguo Quanke Yixue. 2002;5:525-527. |
| 13. | Zhao G, Wang LT, Chen JJ. Effect of anti-fibrosis compound contained serum on procollagen Type I and IV, matrix metalloproteinase and its tissue inhibitor-1 gene expression in HSC-LI90 cell line. Zhongguo ZhongXiYi JieHe ZaZhi. 2004;24:47-50. [PubMed] |
| 14. | Yamashiki M, Nishimura A, Nomoto M, Suzuki H, Kosaka Y. Herbal medicine 'Sho-saiko-to' induces tumour necrosis factor-alpha and granulocyte colony-stimulating factor in vitro in peripheral blood mononuclear cells of patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 1996;11:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Kayano K, Sakaida I, Uchida K, Okita K. Inhibitory effects of the herbal medicine Sho-saiko-to (TJ-9) on cell proliferation and procollagen gene expressions in cultured rat hepatic stellate cells. J Hepatol. 1998;29:642-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Dooley S, Hamzavi J, Breitkopf K, Wiercinska E, Said HM, Lorenzen J, Ten Dijke P, Gressner AM. Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology. 2003;125:178-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 306] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 17. | Liu X, Zhang Z, Yang L, Chen D, Wang Y. Inhibition of the activation and collagen production of cultured rat hepatic stellate cells by antisense oligonucleotides against transforming growth factor-beta 1 is enhanced by cationic liposome delivery. HuaXi YiKe DaXue XueBao. 2000;31:133-135, 142. [PubMed] |
| 18. | Shimizu I. Impact of oestrogens on the progression of liver disease. Liver Int. 2003;23:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Kim KY, Choi I, Kim SS. Progression of hepatic stellate cell activation is associated with the level of oxidative stress rather than cytokines during CCl4-induced fibrogenesis. Mol Cells. 2000;10:289-300. [PubMed] |
| 20. | Reichen J. Liver function and pharmacological considerations in pathogenesis and treatment of portal hypertension. Hepatology. 1990;11:1066-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Deng YQ, Fan XF. Relationship between liver fibrosis criteria and syndrome-type of TCM in patients with non-alcoholic fatty liver. Zhongguo ZhongXiYi JieHe ZaZhi. 2001;21:652-653. [PubMed] |
| 22. | Yao XX, Tang YW, Yao DM, Xiu HM. Effect of Yigan Decoction on the expression of type I, II, III collagen proteins in experimental hepatic in rats. Shijie Huaren Xiaohua Zazhi. 2001;9:263-267. |
| 23. | Tang YW, Yao XX, Yao HS. Observation of ultrastructure and protective effect on Yigankang on liver cells on experimental liver fibrosis rats. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2002;10:76-78. |