Published online Apr 28, 2005. doi: 10.3748/wjg.v11.i16.2438
Revised: August 2, 2004
Accepted: September 24, 2004
Published online: April 28, 2005
AIM: To investigate the effects of eukaryotic expression of plasmid on augmentation of liver regeneration (ALR) in rat hepatic fibrosis and to explore their mechanisms.
METHODS: Ten rats were randomly selected from 50 Wistar rats as normal control group. The rest were administered intraperitoneally with porcine serum twice weekly. After 8 wk, they were randomly divided into: model control group, colchicine group (Col), first ALR group (ALR1), second ALR group (ALR2). Then colchicine ALR recombinant plasmid were used to treat them respectively. At the end of the 4th wk, rats were killed. Serum indicators were detected and histopathological changes were graded. Expression of type I, III, collagen and TIMP-1 were detected by immunohisto-chemistry and expression of TIMP-1 mRNA was detected by semi-quantified RT-PCR.
RESULTS: The histologic examination showed that the degree of the rat hepatic fibrosis in two ALR groups was lower than those in model control group. Compared with model group, ALR significantly reduced the serum levels of ALT, AST, HA, LN, PCIII and IV (P<0.05). Immunohistochemical staining showed that expression of type I, III, collagen and TIMP-1 in two ALR groups was ameliorated dramatically compared with model group (I collagen: 6.94±1.42, 5.80±1.66 and 10.83±3.58 in ALR1, ALR2 and model groups, respectively; III collagen: 7.18±1.95, 4.50±1.67 and 10.25±2.61, respectively; TIMP-1: 0.39±0.05, 0.20±0.06 and 0.53±0.12, respectively, P<0.05 or P<0.01). The expression level of TIMP-1 mRNA in the liver tissues was markedly decreased in two ALR groups compared with model group (TIMP-1 mRNA/β-actin: 0.89±0.08, 0.65±0.11 and 1.36±0.11 in ALR1, ALR2 and model groups respectively, P<0.01).
CONCLUSION: ALR recombinant plasmid has beneficial effects on rat hepatic fibrosis by enhancing regeneration of injured liver cells and inhibiting TIMP-1 expressions.
- Citation: Li Q, Liu DW, Zhang LM, Zhu B, He YT, Xiao YH. Effects of augmentation of liver regeneration recombinant plasmid on rat hepatic fibrosis. World J Gastroenterol 2005; 11(16): 2438-2443
- URL: https://www.wjgnet.com/1007-9327/full/v11/i16/2438.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i16.2438
Hepatic fibrosis is a common pathological process of chronic hepatic disease, which can lead to cirrhosis and increase the risk for hepatocellular carcinoma[1,2]. Advanced fibrosis and cirrhosis were generally considered to be irreversible conditions even after removal of the injurious agent[3]. Over the past 15 years, substantial progress has been made in understanding the cellular and molecular regulation of hepatic fibrosis. It is now clear that the accumulation of extracellular matrix (ECM) in fibrotic diseases of the liver is not a static or unidirectional event but a dynamic and regulated process that is amenable to intervention[4]. At present, the common sense is that cirrhosis could be prevented[5] and hepatic fibrosis could be reversed effectively when the right therapeutic strategy is applied[6]. With the development of the technology of gene therapy and deep study of the mechanism of hepatic fibrosis, the experimental gene therapy of hepatic fibrosis is becoming the main strategy on treating hepatic fibrosis[7-11].
Augmentation of liver regeneration (ALR) was originally cloned from liver tissue of neonatal rats by Hagiya[12] in 1994. Many studies have revealed that ALR appears to be an important regulator of liver regeneration and has trophic effects on regenerating liver and potent antihepatitis effects[13-16].
The present research is to observe the effects of ALR recombinant plasmid on rat hepatic fibrosis. We first established a rat model of immune hepatic fibrosis cirrhosis and then tested the therapeutic effects of ALR recombinant plasmid.
Colchicine has been used in liver diseases as an anti-fibrotic drug[17,18]. We use colchcine here as a positive control treatment.
Restrictive enzymes EcoRI and HindIII were purchased from Promega Corporation (USA); pcDNA3 vector was purchased from Invitrogen Company (USA). The E. coli DH5a was kindly provided by Dr. Yu-Huai Jin (Department of Microbiology of Hebei Medical University). pBV200-ALR plasmid was cloned and constructed by Zhang et al[16]. The primers were synthesized according to Hagiya’s report[12] by Sangon Biological Technology Company (Shanghai, China). The forward: 5’-GCGAAGCTTATGCGGACCCAGAAGC-3’, the reverse: 5’-GCTGAATTCTTAGTCACAGGAGCCCTT-3’. The full-length ALR cDNA was PCR amplified using the primers with pBV220-ALR as the DNA template. The amplified product and pcDNA3 vector DNA were digested respectively with HindIII and EcoRI and then incubated at 75 °C for 10 min. The ALR-pcDNA recombinant plasmid was constructed according to reference[19]. The ALR and pcDNA3 fragment with compatible cohesive terminal were connected in bacteriophage T4 DNA ligation system at 14 °C overnight. The reaction contained T4 DNA ligase 1 μL (3 u), 2´Ligation Buffer 5 μL, 120 μg ALR fragment and 100 μg pcDNA3 fragment, 5 μL ligation mixtures was added to 200 μL E. coli DH5a. Appropriate volume of transformed competent cell onto LB plate containing ampicillin (100 mg/L) was transferred at 37 °C overnight. Bacterial colonies containing ALR plasmid were identified with restriction enzymes (EcoRI, HindIII) and agarose gel electrophoresis.
Fifty Wistar rats, male, weighing 180-200 g, were obtained from Experimental Animal Center of Hebei Medical University, China. The rats were housed (five per cage) in individual cages and subjected to 12 h-day/12 h-night cycle with free access to basic food and water. All animals were treated humanely according to the national guideline for the care of animals in the country.
Taking randomly 10 from 50 Wistar rats as normal control group (N, 10), which were treated with physiological saline. The rest were given an intraperitoneal injection of 0.5 mL of porcine serum according to reference[20], twice a wk for 8 wks. At the 8th wk, the liver pathology proved that the hepatic fibrosis had been established by randomly euthanizing two rats. Then administration of porcine serum was stopped. Those model rats were randomly divided into: model control group (M, 9), colchicine group (Col, 10), first ALR group (ALR1, 9), which were given with porcine serum continuously until the end of experiment), second ALR group (ALR2, 10) and the treatments were administered. The colchicine group was administered with colchicine orally at a dose of 0.14 mg/kg per d; two ALR groups were administered with pcDNA3-ALR recombinant plasmid through caudal vein at a dose of 0.2 mg/kg per wk; normal control group and model control group were administered with physiological saline of 0.5 mL through caudal vein per week. All the administrations lasted for 4 wk.
The rats were euthanized under ether anesthesia by exsanguinations via the abdominal aorta 7 d after the last administration for each group. Serum samples were collected from all rats and livers and spleen were excised.
Liver tissues were fixed in formalin and embedded in paraffin. Hematoxylin and eosin (HE) staining and Masson staining were performed according to the standard procedure. Histologic grade of chronic hepatic fibrosis was determined by a semi-quantitative method based on the criteria described below: grade 0: normal liver, grade 1: few collagen fibrils extended from the central vein and portal tract, grade 2: collagen fibrils extension was apparent but had not yet encompassed the whole lobule, grade 3: collagen fibrils extended into and encompassed the whole lobule, grade 4: diffuse extension of collagen fibrils and pseudo-lobule was formed.
Two pathologists who had no knowledge of their sources and each other’s assessment examined the stained slide independently.
Serum activities of AST and ALT were determined by the Laboratory Department of 4th Affiliated Hospital, Hebei Medical University, China. Serum hyaluronic acid (HA), laminin (LN), types III procollagen (PCIII) and IV collagen concentrations were measured radioimmunologically using a commercial kit (Shanghai Navy Medical Institute, Shanghai, China) according to the manufacturer’s instructions.
Immunohischemistry kit and anti-mouse monoclonal antibodies of Tissue Inhibitors of Metalloproteinase-1 (TIMP-1) were purchased from Boster Biological Technology Ltd (Wuhan, China). Immunohistochemistry was performed according to the method previously described[21].
RNA isolation kits were purchased from Boster Biological Technology Ltd. (Wuhan, China). TIMP-1 mRNA primers were purchased from Sangon Biological Technology Company (Shanghai, China). Total RNA was extracted using an RNA isolation kit, and quantity and quality was detected on a spectrophotometer. Purified RNA 2 mg and primer Oligo (dT) were used for reverse transcription. The primers were: TIMP-1, 482 bp, forward: 5’-TTCGTGGGGACACCAGAAGTC-3’, reverse: 5’-TATCTGGGACCGCAGGGACTG-3’. β-actin, 234 bp, forward: 5’-GGAGAAGATGACCCAGATCA-3’, reverse: 5’-GATCTTCATGAGGTAGTCAG-3’. Amplification conditions included initial denaturation for 5 min at 94 °C, 30 cycles of amplification with denaturation at 94 °C for 45 s, annealing at 61 °C for 45 s, and extension at 72 °C for 1 min. PCR products were analyzed by agarose gel electrophoresis (15 g/L) and visualized by ethidium bromide staining and ultraviolet illumination. Expression of TIMP-1 was scanned by Champ Gel Image Analysis System (Beijing Page Creation Science Company, Beijing, China). The obtained values were related to housekeeping gene β-actin, and the resulting relative ratios were analyzed statistically.
Data were analyzed with SPSS 11.5 software. Quantitative data were presented as mean±SD and compared using one way ANOVA procedure. Frequency data were compared using Ridit procedure.
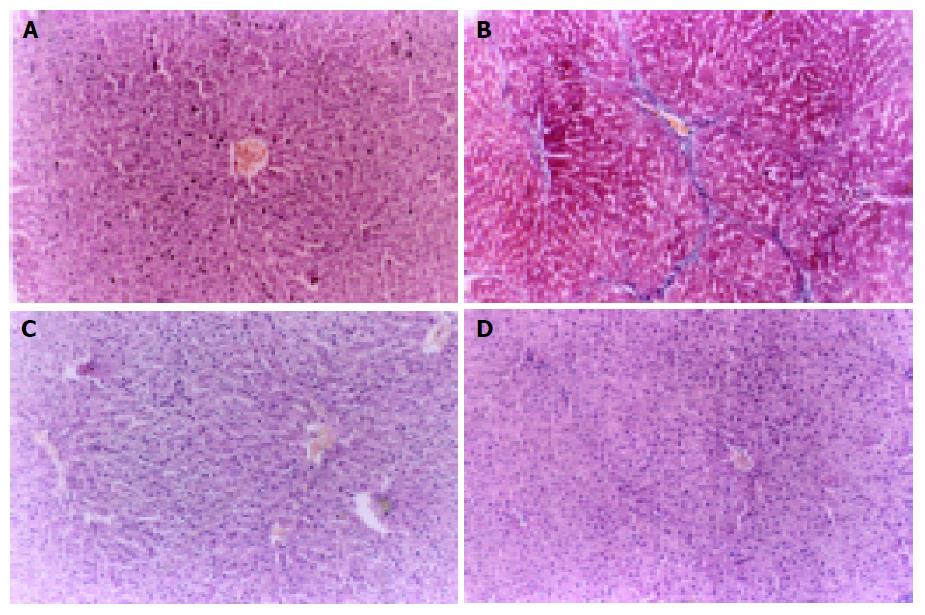
Control livers showed normal lobular architecture with central veins and radiating hepatic cords with irregular sinusoids, and a normal distribution of collagen with a variable amount in portal tracts and a thin rim around central veins (Figure 1A). Livers in model group showed disorderly hepatocyte cords, severe fatty degeneration, spotty or focal necrosis and infiltration of inflammatory cells and collagen deposition extending from central veins or portal tracts, with thick or thin fibrotic septa and even pseudolobuli formation (Figure 1B). Treatment with ALR resulted in apparent amelioration of hepatocyte degeneration, necrosis and infiltration of inflammatory cells and marked reduction in collagen deposition with no obvious pseudolobuli formation in ALR1 group (Figure 1C) and ALR2 group (Figure 1D). Statistical analysis presented significant differences between two ALR groups and model control group in histologic grading, indicating that fibrogenesis in two ALR groups was much less severe than that of model control group (Table 1).
Serum content of ALT, AST, in Col group and two ALR groups was slightly higher than that of normal control group, but significantly lower than that in model control group (P<0.05, Table 2).
Serum content of HA, LN, PCIII (P<0.05) and IV (P<0.01) in Col group and two ALR groups was significantly lower than that in model control group. Serum content of PCIII in ALR2 group was also lower than that in Col group and in ALR1 group (P<0.05, Table 3).
| Group | n | HA (µg/L) | LN (µg/L) | PCIII (µg/L) | IV (µg/L) |
| N | 10 | 338.67±48.24 | 33.71±14.53 | 10.17±2.78 | 36.80±15.04 |
| M | 9 | 435.91±40.31c | 62.50±9.72c | 18.13±7.11c | 125.00±45.06d |
| Col | 10 | 357.90±90.99a | 40.98±13.74a | 11.29±4.42a | 37.78±8.89b |
| ALR1 | 9 | 327.43±111.26a | 36.56±10.01a | 12.60±8.80a | 41.29±23.86b |
| ALR2 | 10 | 319.29±73.91a | 36.04±8.89a | 5.74±2.14aeg | 38.38±16.36b |
These data confirmed the histologic findings that ALR can inhibit hepatic fibrogenesis and improve liver function.
ALR and colchicine can reduce the expression of I, III collagen and TIMP-1 in liver tissue.
Image analysis showed that positive staining index of I and III collagen in model group was the highest (P<0.01) and was evidently decreased in every treated groups (P<0.01).
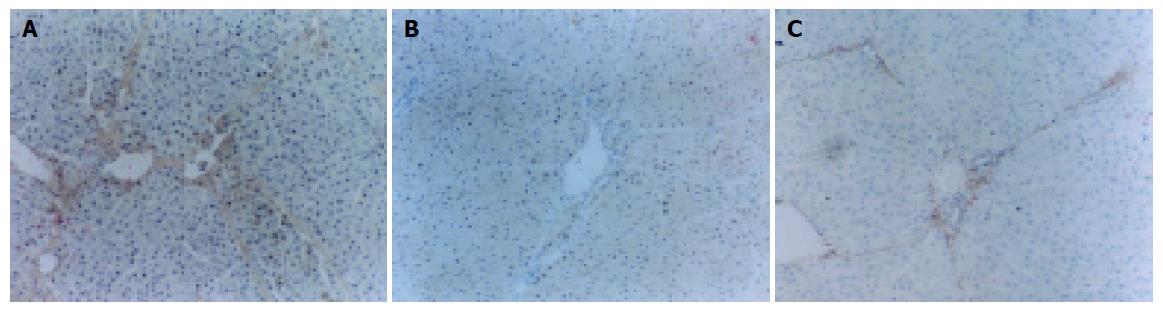
Positive staining of TIMP-1 was found at central vein and Disse’s areas but not at hepatocytes on sections of normal control group, whereas on sections of model control group, the positive staining was seen at interstitial cells, inflammatory cells, impaired hepatocytes as well as normal hepatocytes (Figure 2A). Fibrotic septa were only slightly stained.
Compared with model control group, the staining index of TIMP-1 in colchicine group, ALR1 group (Figure 2B) and ALR2 group (Figure 2C) was markedly decreased (P<0.01). The staining index of III collagen in ALR2 group was lower than in ALR1 group (P<0.05) and the staining index of TIMP-1 in ALR2 group was also markedly lower than in colchicine group and in ALR1 group (P<0.01, Table 4).
TIMP-1 mRNA was detected in normal rat liver, but the expression level was increased significantly in model control group. Compared with model group, the ratio of TIMP-1 mRNA to β-actin in two ALR groups and colchicine group was markedly decreased (P<0.01) and the ratio in ALR2 group was markedly lower than in ALR1 group (P<0.01, Table 4). Thus, the data suggested that ALR and colchicine could reduce the pathologic expression of TIMP-1 in hepatic fibrosis.
Ukei et al[10], found that hepatocyte growth factor (HGF) could promote the proliferation of hepatocytes as a potent mitogen and observably reverse the progression of hepatic fibrosis in rats, indicating that maybe ALR has an effect on hepatic fibrosis.
ALR is a novel cytokine which specifically stimulates hepatic cell proliferation and is able to rescue acute liver failure by inhibition of hepatic natural killer cell activity in acute liver injury[13]. Its complex mechanism might be involved in the synthesis or stability of the nuclear and mitochondrial transcripts that are present in actively regenerating cells. Many data have demonstrated that the administration of exogenous ALR protein can stimulate hepatocyte proliferation and reverse experimental hepatic fibrosis[13-15,21,22].
The present study demonstrated that ALR recombinant plasmid has potent effects for rat hepatic fibrosis based on both histologic examination and functional analysis. Its effects are even greater than the effects of colchicine in detecting the expression of TIMP-1. HA, LN, PCIII and IV have been found to be ideal serum markers of hepatic fibrosis. We detected that the serum content of HA, LN, PCIII and IV in two ALR group is decreased markedly compared with model group. In addition, ALR recombinant plasmid can reduce heightened ALT and AST, indicating that they can promote the repair of injured liver cell.
An ideal anim model should be very similar to the characteristics of human disease. Here the rats in ALR1 group were stimulated continuously by pathogenic factor (administered intraperitoneally with porcine serum) until the end of the experiment. We think that the pathogenic stimulating would cease even if patients were administered with drugs in lots of cases. In the present study, model control group, two ALR groups represent respectively spontaneous resolution of hepatic fibrosis, therapy plus removing pathogeny (ALR2 group) and therapy but not removing pathogeny (ALR1 group). The therapeutic effects of ALR in ALR2 group are more ideal than in ALR1 group through detecting the expression of III collagen, TIMP-1 and TIMP-1 mRNA, which suggests that removing pathogeny can achieve more ideal effects for treating hepatic fibrosis.
Hepatic fibrosis is the process of excessive deposition of collagen and other ECM component. Some ECM deposition is necessary for wound healing to provide strength and temporary structure to damaged tissue; however, if not limited, it can be pathologic[23]. The increase of ECM synthesis and decrease of ECM degradation will result in excess deposition of ECM in liver. The decrease of ECM degradation was the primary reason for the excess deposition of ECM in the late stage. The metalloproteinases (MMPs) played a leading role during the degradation of ECM[24-26]. MMPs were a group of zinc-ion dependent enzymes, which created conditions for further degradation of other proteinases through reducing the stability of helical structure of collagen and changing the secondary structure of substrates. TIMPs were a group of polypeptides with the ability of inhibiting the function of MMPs. Research work showed that TIMP can be divided into four classes: TIMP-1, TIMP-2, TIMP-3, and TIMP-4. However, only TIMP-1 and TIMP-2 could be detected in liver[27-29]. The expression of TIMP-1 was more obvious than that of TIMP-2. All these indicated that TIMP-1 did play an important role in the development of liver fibrosis and cirrhosis. At present, it was found that TIMP-1 in the injured liver increased early and obviously, and many researchers thought that TIMP-1 was a very important promoting factor in the process of hepatic fibrosis[30-32]. This has important implications for the future development of therapeutic antifibrotic strategies in the liver[31,32]. It may be the basic mechanism of reversing hepatic fibrosis and a leading strategy of treating hepatic fibrosis to suppress the expression of TIMP-1 mRNA.
In normal liver, the collagen types I, III account for about 80% of the total collagen of liver, while it rises up to more than 95% in fibrotic liver. The collagen type I covers about 60-70% of the total collagen of fibrotic liver, and type III is 20-30%[33,34]. Therefore, collagen I, III are regarded as the important parameters to reflect the metabolism of collagen, and thus we can judge the therapeutic effect of anti-fibrosis strategies[35,36]. The content of collagen I, III was lower in the ALR group than that in model group. At the same time, we found that TIMP-1 and TIMP-1 mRNA were expressed obviously in model group and TIMP-1 level in ALR groups were markedly decreased.
These data showed that ALR recombinant plasmid can increase the degradating capacity of collagen I, III, decrease the deposition of ECM and the expression of TIMP-1 in pathologic liver tissue and thus reverse the hepatic fibrosis induced by porcine serum administration. This may be the molecular mechanism of ALR. ALR gene therapy may be potentially useful for the treatment of patients with liver cirrhosis.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Miyazawa K, Moriyama M, Mikuni M, Matsumura H, Aoki H, Shimizu T, Yamagami H, Kaneko M, Shioda A, Tanaka N. Analysis of background factors and evaluation of a population at high risk of hepatocellular carcinoma. Intervirology. 2003;46:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Nagao Y, Fukuizumi K, Kumashiro R, Tanaka K, Sata M. The prognosis for life in an HCV hyperendemic area. Gastroenterology. 2003;125:628-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Lee HS, Huang GT, Chen CH, Chiou LL, Lee CC, Yang PM, Chen DS, Sheu JC. Less reversal of liver fibrosis after prolonged carbon tetrachloride injection. Hepatogastroenterology. 2001;48:1312-1315. [PubMed] |
| 4. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1597] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 5. | Riley TR, Bhatti AM. Preventive strategies in chronic liver disease: part II. Cirrhosis. Am Fam Physician. 2001;64:1735-1740. [PubMed] |
| 6. | Brenner DA. Signal transduction during liver regeneration. J Gastroenterol Hepatol. 1998;13 Suppl:S93-S95. [PubMed] |
| 7. | Garcia-Bañuelos J, Siller-Lopez F, Miranda A, Aguilar LK, Aguilar-Cordova E, Armendariz-Borunda J. Cirrhotic rat livers with extensive fibrosis can be safely transduced with clinical-grade adenoviral vectors. Evidence of cirrhosis reversion. Gene Ther. 2002;9:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Salgado S, Garcia J, Vera J, Siller F, Bueno M, Miranda A, Segura A, Grijalva G, Segura J, Orozco H. Liver cirrhosis is reverted by urokinase-type plasminogen activator gene therapy. Mol Ther. 2000;2:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science. 2000;287:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 303] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 10. | Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 471] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 11. | Ueno H, Sakamoto T, Nakamura T, Qi Z, Astuchi N, Takeshita A, Shimizu K, Ohashi H. A soluble transforming growth factor beta receptor expressed in muscle prevents liver fibrogenesis and dysfunction in rats. Hum Gene Ther. 2000;11:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Hagiya M, Francavilla A, Polimeno L, Ihara I, Sakai H, Seki T, Shimonishi M, Porter KA, Starzl TE. Cloning and sequence analysis of the rat augmenter of liver regeneration (ALR) gene: expression of biologically active recombinant ALR and demonstration of tissue distribution. Proc Natl Acad Sci USA. 1994;91:8142-8146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Tanigawa K, Sakaida I, Masuhara M, Hagiya M, Okita K. Augmenter of liver regeneration (ALR) may promote liver regeneration by reducing natural killer (NK) cell activity in human liver diseases. J Gastroenterol. 2000;35:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Polimeno L, Capuano F, Marangi LC, Margiotta M, Lisowsky T, Ierardi E, Francavilla R, Francavilla A. The augmenter of liver regeneration induces mitochondrial gene expression in rat liver and enhances oxidative phosphorylation capacity of liver mitochondria. Dig Liver Dis. 2000;32:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Yang X, Wang A, Zhou P, Wang Q, Wei H, Wu Z, He F. Protective effect of recombinant human augmenter of liver regeneration on CCl4-induced hepatitis in mice. Chin Med J (Engl). 1998;111:625-629. [PubMed] |
| 16. | Zhang LM, Liu DW, Yang J, Li Q. Construction of eukaryotic express plasmid of rat augmenter of liver regeneration gene and effect on acute hepatic failure in rats. Hebei Yike Daxue Xuebao. 2004;25:68-70. |
| 17. | Lee SJ, Kim YG, Kang KW, Kim CW, Kim SG. Effects of colchicine on liver functions of cirrhotic rats: beneficial effects result from stellate cell inactivation and inhibition of TGF beta1 expression. Chem Biol Interact. 2004;147:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Das D, Pemberton PW, Burrows PC, Gordon C, Smith A, McMahon RF, Warnes TW. Antioxidant properties of colchicine in acute carbon tetrachloride induced rat liver injury and its role in the resolution of established cirrhosis. Biochim Biophys Acta. 2000;1502:351-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Peng D, Qian C, Sun Y, Barajas MA, Prieto J. Transduction of hepatocellular carcinoma (HCC) using recombinant adeno-associated virus (rAAV): in vitro and in vivo effects of genotoxic agents. J Hepatol. 2000;32:975-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Shiga A, Shirota K, Nishita T, Nomura Y. Study on the pathogenesis of porcine serum-induced liver fibrosis in rats with special reference to the effects of hypertension. J Vet Med Sci. 1998;60:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Giorda R, Hagiya M, Seki T, Shimonishi M, Sakai H, Michaelson J, Francavilla A, Starzl TE, Trucco M. Analysis of the structure and expression of the augmenter of liver regeneration (ALR) gene. Mol Med. 1996;2:97-108. [PubMed] |
| 22. | Polimeno L, Margiotta M, Marangi L, Lisowsky T, Azzarone A, Ierardi E, Frassanito MA, Francavilla R, Francavilla A. Molecular mechanisms of augmenter of liver regeneration as immunoregulator: its effect on interferon-gamma expression in rat liver. Dig Liver Dis. 2000;32:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Vaillant B, Chiaramonte MG, Cheever AW, Soloway PD, Wynn TA. Regulation of hepatic fibrosis and extracellular matrix genes by the th response: new insight into the role of tissue inhibitors of matrix metalloproteinases. J Immunol. 2001;167:7017-7026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Herbst H, Wege T, Milani S, Pellegrini G, Orzechowski HD, Bechstein WO, Neuhaus P, Gressner AM, Schuppan D. Tissue inhibitor of metalloproteinase-1 and -2 RNA expression in rat and human liver fibrosis. Am J Pathol. 1997;150:1647-1659. [PubMed] |
| 25. | Ulrich D, Lichtenegger F, Eblenkamp M, Repper D, Pallua N. Matrix metalloproteinases, tissue inhibitors of metalloproteinases, aminoterminal propeptide of procollagen type III, and hyaluronan in sera and tissue of patients with capsular contracture after augmentation with Trilucent breast implants. Plast Reconstr Surg. 2004;114:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Li YL, Sato M, Kojima N, Miura M, Senoo H. Regulatory role of extracellular matrix components in expression of matrix metalloproteinases in cultured hepatic stellate cells. Cell Struct Funct. 1999;24:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci (Lond). 1997;92:103-112. [PubMed] |
| 28. | Iredale JP. Tissue inhibitors of metalloproteinases in liver fibrosis. Int J Biochem Cell Biol. 1997;29:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 122] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Arthur MJ, Iredale JP, Mann DA. Tissue inhibitors of metalloproteinases: role in liver fibrosis and alcoholic liver disease. Alcohol Clin Exp Res. 1999;23:940-943. [PubMed] |
| 30. | Flisiak R, Maxwell P, Prokopowicz D, Timms PM, Panasiuk A. Plasma tissue inhibitor of metalloproteinases-1 and transforming growth factor beta 1--possible non-invasive biomarkers of hepatic fibrosis in patients with chronic B and C hepatitis. Hepatogastroenterology. 2002;49:1369-1372. [PubMed] |
| 31. | Yoshiji H, Kuriyama S, Miyamoto Y, Thorgeirsson UP, Gomez DE, Kawata M, Yoshii J, Ikenaka Y, Noguchi R, Tsujinoue H. Tissue inhibitor of metalloproteinases-1 promotes liver fibrosis development in a transgenic mouse model. Hepatology. 2000;32:1248-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 198] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 32. | Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 828] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 33. | Louis H, Le Moine A, Quertinmont E, Peny MO, Geerts A, Goldman M, Le Moine O, Devière J. Repeated concanavalin A challenge in mice induces an interleukin 10-producing phenotype and liver fibrosis. Hepatology. 2000;31:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Kovalovich K, DeAngelis RA, Li W, Furth EE, Ciliberto G, Taub R. Increased toxin-induced liver injury and fibrosis in interleukin-6-deficient mice. Hepatology. 2000;31:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 243] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 36. | Arthur MJ. Degradation of matrix proteins in liver fibrosis. Pathol Res Pract. 1994;190:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |