Published online Apr 28, 2005. doi: 10.3748/wjg.v11.i16.2398
Revised: June 9, 2004
Accepted: August 12, 2004
Published online: April 28, 2005
AIM: The β-catenin has been recognized as a critical member of the Wnt signaling pathway and plays an important role in the generation/differentiation of many tissues. Inappropriate activation of this pathway has been implicated in carcinogenesis. The mechanism underlying the development as well as its prognosis of hepatocellular carcinoma (HCC) has remained unclear. The purpose of this study is to analyze the expression of β-catenin in HCC in relation to histological grades and viral hepatitis backgrounds.
METHODS: Thirty-two sections were selected at random from autopsy and surgical cases of HCC. Immuohistologically, the location and positivity of β-catenin expression in HCC was examined.
RESULTS: Normal hepatocytes did not express β-catenin. In 78% of HCC β-catenin was expressed at the membrane of the cells, with or without cytoplasmic and/or nuclear expression. The tumor cells with well- and moderately-differentiated grades expressed frequently at the membrane and cytoplasm compared with poorly-differentiated type. Nuclear expression of β-catenin was prone to occur in the tumor cells of poorly-differentiated grade. There were 15% of hepatitis C virus (HCV) backgrounds with nuclear expression. In contrast, there was 38% with nuclear expression in hepatitis B virus (HBV) backgrounds. In nonB-nonC hepatitis, no case expressed nuclear β-catenin.
CONCLUSION: The β-catenin expression in HCC cells was heterogenous among types of hepatitis viral infection. Wnt signaling pathway might be deeply involved in less-differentiated HCC and HBV background.
- Citation: Tien LT, Ito M, Nakao M, Niino D, Serik M, Nakashima M, Wen CY, Yatsuhashi H, Ishibashi H. Expression of β-catenin in hepatocellular carcinoma. World J Gastroenterol 2005; 11(16): 2398-2401
- URL: https://www.wjgnet.com/1007-9327/full/v11/i16/2398.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i16.2398
Hepatocellular carcinoma (HCC) is a major type of primary liver cancer and one of the rare human neoplasms etiologically linked to viral factors. Chronic infections with the hepatitis B virus (HBV) and the hepatitis C virus (HCV) have been implicated in about 80% of cases worldwide. However, the molecular mechanisms underlying their development are still poorly understood.
HCCs display gross genomic alterations, including DNA rearrangements associated with HBV DNA integration, loss of heterozygosity, and, less importantly, chromosomal amplifications and loss of imprinting[1]. Many genes with somatic mutations have been identified in these tumors. Most frequently involved genes are tumor suppressor genes such as p53, β-catenin, and retinoblastoma genes[2-4].
β-Catenin is a structure protein in the cadherin mediated cell-cell adhesive system as a regulator[5], and plays an important role in the generation/differentiation of tissues as well as in the repair of normal tissues. It is also known to act as a mediator in the Wingless/Wnt signal transduction pathway[6]. In HCC, accumulation of β-catenin was present in the early stage of HCC[7,8]. The previous studies investigated the correlation between β-catenin expression and the differentiation grades of HCC, and prognostic roles of β-catenin expression in HCC[9-11].
The purpose of this study is to investigate the expression of β-catenin in HCC in relation to histological grades and to viral hepatitis backgrounds.
Liver tissue sections were obtained at random of 32 sections from 15 autopsy cases and 13 surgical cases. They consisted of 20 males and 8 females. Age ranged from 36 to 86 years with an average of 64. The diagnosis was confirmed histopathologically in all cases, based mainly on examination of sections stained with H&E.
The sections were stained immunohistochemically by the avidin-biotin complex method for β-catenin antibody (Mouse IgG1, BD Transduction Laboratories - BD Biosciences, SanJose, California, USA) with dilution of 1:200. For immunostaining, the sections were deparaffinized, washed with phosphate-buffered saline (PBS, pH 7.4) in 10 min, soaked in sodium citrate buffer (pH 6.0) and heated in microwave at 97 °C in 15 min for antigen retrieval. It was then allowed to cool at room temperature before being immersed into 0.3% H2O2/methanol to block endogenous peroxidase activity. The sections were pre-incubated with 10% normal bovine serum to prevent non-specific binding. Primary antibodies were incubated for 2 h at room temperature. Secondary antibodies, anti-mouse IgG was applied for 30 min, followed by incubation with avidin-peroxidase for 10 min and visualized with diaminobenzidine (DAB), rinsed and soaked in PBS for 3-5 min, thrice after each step. They were counterstained with Mayer hematoxylin.
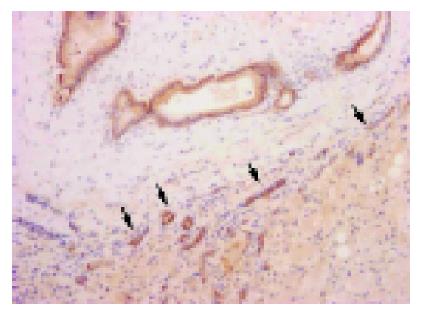
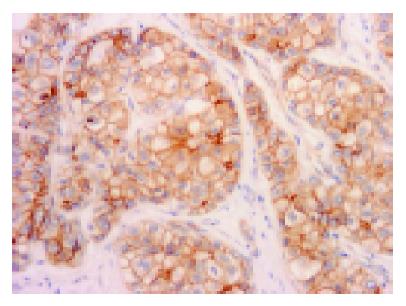
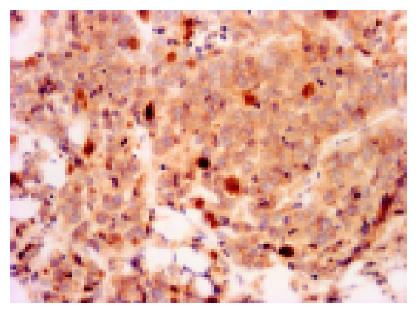
For the evaluation of immunohistochemical staining of β-catenin, the constitutional expression on the cell membrane of bile duct at non-cancerous area was used as positive control (Figure 1). The membranous expression (Figure 2) was evaluated as (+) when at least one-third section of it was expressed or strong expression if less than one-third and (-) when it was unexpressed or less than one-third section was weakly expressed. The cytoplasmic expression was evaluated as + when it was stronger than the expression in non-cancerous areas, and (-) when the expression was the same as in non-cancerous area. In the nucleus, the expression (Figure 3) was evaluated as + when it was found clearly in any portion. All the results were observed and agreed by two pathologists (Dr. Ito and Dr. Wen).
There were 20 sections with hepatitis C virus background, eight with hepatitis B virus, three with nonB-nonC viral hepatitis, and one section with non-hepatitis infection. Eleven sections were well-differentiated type, 17 were moderately-differentiated type, and four sections were poorly-differentiated type. In well-differentiated cases, their backgrounds consisted of two with viral hepatitis B, six with viral hepatitis C, and three with nonB-nonC viral hepatitis. In moderately-differentiated sections, their backgrounds were three sections with viral hepatitis B, 13 sections with viral hepatitis C, and one section with nonB-nonC viral hepatitis. In poorly-differentiated cases, their backgrounds were three with viral hepatitis B and one section with viral hepatitis C.
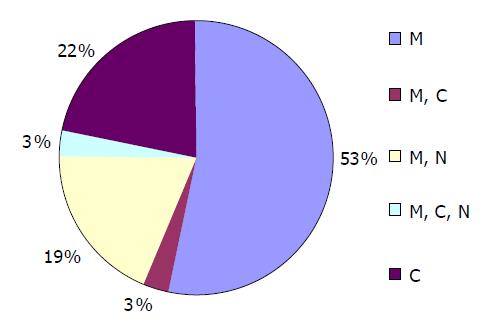
Most sections (25 of 32 sections: 78%) expressed at the membrane of the cells. Among them, 17 of 32 sections (53%) were only expressed at the membrane (Figure 2). For cytoplasmic expression, there were 9 of 32 cases (28%), but 7 of 32 sections (22%) were only expressed at the cytoplasm and two cases were associated with membranous and/or nuclear expression. There were 7 of 32 (22%) sections with nuclear expression (Figure 3) and there were no cases with nuclear expression alone. Figure 4 shows percentages of immunocytochemical expression pattern in the tumor cells.
| M (%) | M+N (%) | M+C (%) | M+N+C (%) | C(%) | |
| HBV | 49 (4/8) | 38 (3/8) | 13 (1/8) | ||
| HCV | 50 (10/20) | 10 (2/20) | 5 (1/20) | 5 (1/20) | 30 (6/20) |
| NonB-nonC | 100 (3/3) |
There were eight sections with HBV background which were mainly β-catenin expression of membranous type, in which three sections were both membranous and nuclear expressions. There were 20 sections with HCV background in which six sections were cytoplasmic. Two sections were membranous and nuclear type. One section was membranous and cytoplasmic expression. One section was membrano-cytoplasm-nuclear expression and 10 sections were membranous expression of β-catenin. The sections with nonB-nonC expressed only membranous type. Overall the nuclear localization of β-catenin was highly encountered in HBV background.
| M (%) | M+N (%) | M+C (%) | M+N+C (%) | C (%) | |
| Well | 46 (5/11) | 18 (2/11) | 9 (1/11) | 27 (3/11) | |
| Moderate | 65 (11/17) | 12 (2/17) | 23 (4/17) | ||
| Poor | 25 (1/4) | 50 (2/4) | 25 (1/4) |
For well-differentiated grade, there were five sections with membranous expression, three sections with cytoplasmic expression, two sections with membrano-nuclear expression, and one section with membrano-cytoplasmic expression. For moderately-differentiated grade, there were 11 sections with membranous expression, four sections with cytoplasmic expression, and two sections with membrano-nuclear expressions. For poorly-differentiated grade, there was one section with membranous expression, two sections with membrano-nuclear expression, and one section with membrano-cytoplasm-nuclear expression.
There were 7 of 32 sections (22%) which expressed the nuclear type, in which three sections were encountered in poorly-differentiated grade, two sections in moderately-differentiated grade, and two sections in well-differentiated grade. In each differentiation grade, nuclear expression was highly detected in poorly-differentiated type (3 of 4 sections, 75%) compared with well (2 of 11 sections, 18%) and moderately-differentiated types (2 of 17 sections, 12%).
Prevalence and localization of β-catenin nuclear expression and mutation varies among previous reports[7,9-15]. Also prognostic implication of β-catenin expression in hepatocarcinogenesis is inconsistent[9-11,14,15]. Some reports suggest better prognosis in cases with nuclear expression and mutation[10,16], but most reports indicate tumor progression and tumor cell proliferation[9,11,15].
In HCC, accumulation of β-catenin was present in the early stage of HCC. Most authors have described that β-catenin is strongly expressed on the membrane of HCC cells[7,9-11]. Suzuki et al[7] showed that β-catenin expression in nodule-in-nodule HCC is 41.7% at the membrane and 41.7% in the cytoplasm of the cells. Inagawa et al[9] noted that about 61% exhibited increased membranous and/or cytoplasmic expression. Hey-Chi Hsu et al[10] examined 366 cases of multifocal HCCs and stained 282 cases immunochemically for β-catenin, in which 212 cases (57.9%) expressed at the cell membrane alone and 70 cases (19.1%) expressed scattered nuclear expressions. We have documented 78% β-catenin expression at the membrane with or without cytoplasmic and/or nuclear expression, in which 53% of them expressed only at the membrane of the cells. The tumor cells with well- and moderately-differentiated grade expressed at the membrane and cytoplasm for β-catenin. Nuclear expression of β-catenin occurred in moderately- and poorly-differentiated grades, although having no section with nuclear expression alone. In viral infection background, HBV-infected HCC expressed nuclear translocation in high prevalence. These findings suggest that nuclear expression of β-catenin is likely to be induced in less-differentiated type and HBV backgroud. Nuclear expression of β-catenin implies more important roles in view of tumor progression than membranous and cytoplasmic expression. β-catenin can enter the nucleus by binding the T-cell factor and lymphoid-enhancer factor family of DNA binding proteins, and regulates transcription of target genes, such as cyclin D1 and c-myc. Both c-mys and cyclin D1 are involved in the transition between the G1-S check-point of the cell cycle and do so by influencing the activity of retinoblastoma tumor-suppressor pRB[17,18].
Cyclin D1 is a major regulator of the progressing of cells into proliferation stage of the cell cycle. Increased β-catenin levels may promote neoplastic conversion by triggering cyclin D1 gene expression and consequently uncontrolled progression into the cell cycle. Activation of cyclin D1 and disruption of the Rb pathway are also commonly involved in liver tumorigenesis[18]. In our cases cyclin D1 was expressed only in poorly-differentiated HCCs (data not shown). In less-differentiated HCCs, cell proliferation might be induced via β-catenin/cyclin D1 signal pathway[10,19]. But the prevalent type of HCC, well- and moderately-differentiated type, does not seem to be involved in cyclin D1 overexpression. One study suggests that without cyclin D1 activation the β-catenin-related cell proliferation exists in hepatocarcinogenesis[9,20]. And the study on embryonic development indicated that β-catenin might not regulate cyclin D1 during liver development[21].
Statistical analysis clearly showed a distinction between the gene expression profiles of HCV and HBV-related HCC[22]. HBV-associated HCC exhibited involvement of different cellular pathways, those controlling apoptosis, p53 signaling and G1/S transition. In HCV-related HCC a more heterogenous pattern with an over-expression of the TGF-beta induced gene was identified. It is still unclear as to whether the gene expression profile in HCV or HBV-related HCC exhibits a degree of specificity[22].
By mutational analysis of β-catenin gene, Hey-Chi Hsu et al[10] indicated that mutation plays a more important role in the tumorigenesis of non-HBV-related HCC than in HBV-related HCC and different types of β-catenin mutations reflect different etiologies of carcinogenesis in specific tissue. In this study, viral hepatitis backgrounds were 63% of HCV and 25% of HBV. This prevalence is similar to that of previous studies. There are differences of expression pattern for β-catenin between B and C viral hepatitis backgrounds. Nuclear translocation was highly observed in HBV background. These results suggest that the expression of β-catenin is influenced by virus infections and carcinogenesis of HBV and HCV infections are different. Integrated hepatitis B virus (HBV) DNA is present in many HCC, suggesting that HBV has a direct oncogenic effect through interaction with transformation-associated genes. The HBX protein of hepatitis B virus is thought to contribute to the development of carcinoma by disruption of intercellular adhesion. β-catenin was tyrosine-phosphorylated in a Src-dependent manner in HBX-expressing cells. Tyrosine phosphorylation of β-catenin by Src kinase results in an increase in its free cytosolic pool. Recent study suggests that HBX induces the stabilization and subsequent nuclear translocation of β-catenin by activating Src kinase and GSK3β suppression.
The overall cytological expression pattern was not consistent with previous reports, suggesting that ethnical and district differences are significant in Wnt signaling pathway in hepatocarcinogenesis. More studies are warranted to understand the expression of β-catenin which effects histological grade and viral hepatitis background in HCC better.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Calvisi DF, Factor VM, Ladu S, Conner EA, Thorgeirsson SS. Disruption of beta-catenin pathway or genomic instability define two distinct categories of liver cancer in transgenic mice. Gastroenterology. 2004;126:1374-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Ozturk M. Genetic aspects of hepatocellular carcinogenesis. Semin Liver Dis. 1999;19:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Prange W, Breuhahn K, Fischer F, Zilkens C, Pietsch T, Petmecky K, Eilers R, Dienes HP, Schirmacher P. Beta-catenin accumulation in the progression of human hepatocarcinogenesis correlates with loss of E-cadherin and accumulation of p53, but not with expression of conventional WNT-1 target genes. J Pathol. 2003;201:250-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Torbenson M, Kannangai R, Abraham S, Sahin F, Choti M, Wang J. Concurrent evaluation of p53, beta-catenin, and alpha-fetoprotein expression in human hepatocellular carcinoma. Am J Clin Pathol. 2004;122:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Wei Y, Van Nhieu JT, Prigent S, Srivatanakul P, Tiollais P, Buendia MA. Altered expression of E-cadherin in hepatocellular carcinoma: correlations with genetic alterations, beta-catenin expression, and clinical features. Hepatology. 2002;36:692-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Cui J, Zhou X, Liu Y, Tang Z, Romeih M. Wnt signaling in hepatocellular carcinoma: analysis of mutation and expression of beta-catenin, T-cell factor-4 and glycogen synthase kinase 3-beta genes. J Gastroenterol Hepatol. 2003;18:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Suzuki T, Yano H, Nakashima Y, Nakashima O, Kojiro M. Beta-catenin expression in hepatocellular carcinoma: a possible participation of beta-catenin in the dedifferentiation process. J Gastroenterol Hepatol. 2002;17:994-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Harada N, Oshima H, Katoh M, Tamai Y, Oshima M, Taketo MM. Hepatocarcinogenesis in mice with beta-catenin and Ha-ras gene mutations. Cancer Res. 2004;64:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Inagawa S, Itabashi M, Adachi S, Kawamoto T, Hori M, Shimazaki J, Yoshimi F, Fukao K. Expression and prognostic roles of beta-catenin in hepatocellular carcinoma: correlation with tumor progression and postoperative survival. Clin Cancer Res. 2002;8:450-456. [PubMed] |
| 10. | Hsu HC, Jeng YM, Mao TL, Chu JS, Lai PL, Peng SY. Beta-catenin mutations are associated with a subset of low-stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am J Pathol. 2000;157:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 229] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Buendia MA. Genetics of hepatocellular carcinoma. Semin Cancer Biol. 2000;10:185-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 245] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Nhieu JT, Renard CA, Wei Y, Cherqui D, Zafrani ES, Buendia MA. Nuclear accumulation of mutated beta-catenin in hepatocellular carcinoma is associated with increased cell proliferation. Am J Pathol. 1999;155:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 229] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Huang H, Fujii H, Sankila A, Mahler-Araujo BM, Matsuda M, Cathomas G, Ohgaki H. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol. 1999;155:1795-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 205] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 14. | Wong CM, Fan ST, Ng IO. beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 2001;92:136-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Kondo Y, Kanai Y, Sakamoto M, Genda T, Mizokami M, Ueda R, Hirohashi S. Beta-catenin accumulation and mutation of exon 3 of the beta-catenin gene in hepatocellular carcinoma. Jpn J Cancer Res. 1999;90:1301-1309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Fujito T, Sasaki Y, Iwao K, Miyoshi Y, Yamada T, Ohigashi H, Ishikawa O, Imaoka S. Prognostic significance of beta-catenin nuclear expression in hepatocellular carcinoma. Hepatogastroenterology. 2004;51:921-924. [PubMed] |
| 17. | Edamoto Y, Hara A, Biernat W, Terracciano L, Cathomas G, Riehle HM, Matsuda M, Fujii H, Scoazec JY, Ohgaki H. Alterations of RB1, p53 and Wnt pathways in hepatocellular carcinomas associated with hepatitis C, hepatitis B and alcoholic liver cirrhosis. Int J Cancer. 2003;106:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Lévy L, Renard CA, Wei Y, Buendia MA. Genetic alterations and oncogenic pathways in hepatocellular carcinoma. Ann N Y Acad Sci. 2002;963:21-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci USA. 1999;96:5522-5527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1701] [Cited by in RCA: 1782] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 20. | Shang XZ, Zhu H, Lin K, Tu Z, Chen J, Nelson DR, Liu C. Stabilized beta-catenin promotes hepatocyte proliferation and inhibits TNFalpha-induced apoptosis. Lab Invest. 2004;84:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Monga SP, Monga HK, Tan X, Mulé K, Pediaditakis P, Michalopoulos GK. Beta-catenin antisense studies in embryonic liver cultures: role in proliferation, apoptosis, and lineage specification. Gastroenterology. 2003;124:202-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Delpuech O, Trabut JB, Carnot F, Feuillard J, Brechot C, Kremsdorf D. Identification, using cDNA macroarray analysis, of distinct gene expression profiles associated with pathological and virological features of hepatocellular carcinoma. Oncogene. 2002;21:2926-2937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |