Published online Apr 21, 2005. doi: 10.3748/wjg.v11.i15.2351
Revised: April 25, 2004
Accepted: April 29, 2004
Published online: April 21, 2005
AIM: To investigate the occurrence of cellular src (c-src) activating mutation at codon 531 in colorectal cancer patients from Chinese mainland.
METHODS: Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay followed by sequencing and single-strand conformation polymor-phism analysis were carried out to screen 110 samples of primary colorectal cancer and 20 colorectal liver metastases.
RESULTS: Only one sample showed PCR-RFLP-positive results and carried somatic codon 531 mutations. No additional mutation of c-src exon 12 was found.
CONCLUSION: c-src codon 531 mutation in colorectal cancer is not the cause of c-src activation.
- Citation: Tan YX, Wang HT, Zhang P, Yan ZH, Dai GL, Wu MC, Wang HY. c-src activating mutation analysis in Chinese patients with colorectal cancer. World J Gastroenterol 2005; 11(15): 2351-2353
- URL: https://www.wjgnet.com/1007-9327/full/v11/i15/2351.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i15.2351
Liver metastasis is one of the main causes of death of colorectal cancer patients. Although it is well known that tumor metastasis is a multi-factorial and multi-step process, the exact mechanisms underlying metastasis of colorectal cancer are still largely unclear. Elevated expression and activity of the src family tyrosine kinase are often associated with progression and metastasis of human colorectal cancer[1]. As for the mechanism of src activation, Irby et al[2], reported an activating c-src mutation, C-T mutation in codon 531 (located at exon 12), in 12% of advanced human colon cancers[2]. But the following likewise screens of this kind of mutations in four groups all had negative results[3-6]. It is still quite uncertain whether codon 531 activating mutation is a cause of c-src activation in advanced colorectal cancers.
In this study, we investigated 110 primary colorectal cancers and 20 colorectal liver metastasis patients from China mainland for this c-src specific mutation.
Primary colorectal tumors and corresponding normal tissues and blood samples were obtained from a total of 110 Chinese patients (median age of 52 years, ranging from 36 to 72 years) with primary colorectal cancer during surgery at Changhai Hospital and Changzheng Hospital (both in Shanghai, China) from May 2003 to December 2003. The tumors were classified as Duke stage A in 21, B in 26, C in 38 and D in 25. Twenty liver metastases with colorectal origin were from Shanghai Eastern Hepatobiliary Surgery Hospital. Tissue samples were stored at -80 °C until DNA extraction.
Genomic DNA was extracted as previously described[7]. DNAs from all 130 samples served as templates for PCR (Perkin-Elmer) with primers set to amplify exon 12 of c-src. The sequences of primers were as follows: forward, 5’-ACAGGGATGGTGAACCGCGA-3’ and reverse, 5’-ATCCAAGCCGAGAAGCCGGT-3’.
After DNA extraction (QIAquick PCR purification kit, Qiagen), PCR products were digested with 5 Units of restriction enzyme Sca I overnight. Plasmid DNAs bearing src 531 mutation created by using overlap extension PCR[8] were used as positive controls. The possible digestion-positive samples were cloned into T-vector (pGEM®-T vector, Promega) and sequenced automatically (Perkin Elmer-Roche, Banchburg, NJ).
PCR products of genomic DNA were screened for mutations by single-strand conformational polymorphism (SSCP) analysis exactly as previously described[9].
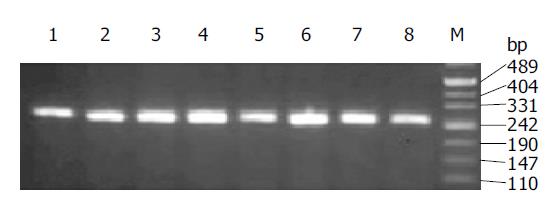
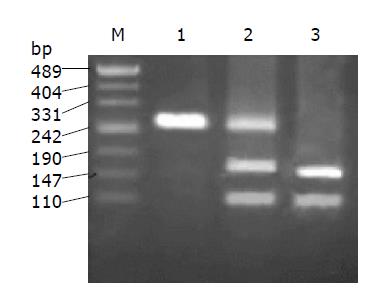
PCR with primers devised here generated a 266-bp fragment, which was confirmed to be exon 12 of c-src by direct sequencing (Figure 1). Only one sample (Figure 2) was positive by RFLP analysis among the 110 samples of colorectal cancer and 20 liver metastases in this study. This No. 73 sample could only be partially digested by 5U Sca I even after overnight digestion, implying that the corresponding DNA template was a mixture of mutated and wild type clones.
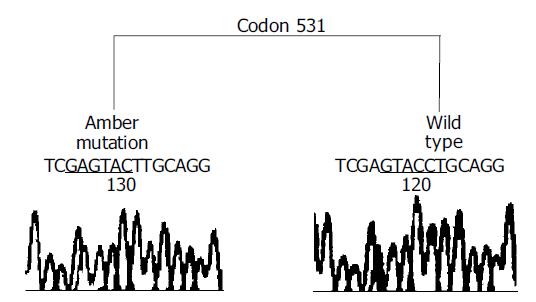
This positive PCR product was cloned into T vector, and 20 transformants were selected for sequencing. The result confirmed the presence of 531 C-T mutation and the ratio of mutated clones vs wild type clones was 8:12 (Figure 3). To further know whether it was a somatic or germ line mutation, we analyzed its paired normal samples and blood likewise, but could not find any mutation (Figure 2).
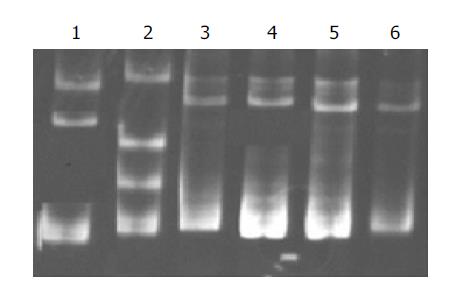
Additionally, SSCP was carried out to screen possible other mutations of c-src in exon 12. No aberrant band was found except for No. 73 sample and positive control (Figure 4).
Cellular src (c-src) is a human homolog of the Rous sarcoma viral oncogene, v-src. c-src has been implicated in the development and progression of numerous human cancers, including colorectal cancer[10]. c-src was shown to be dramatically activated in colorectal cancer[11,12]. Irby et al[2], reported src point mutation at codon 531 truncated c-src C-terminal to the negatively regulatory kinase phosphorylation site at Tyr 530 and thereby activated c-src. This was the first report of activating c-src mutation related to the progression of colorectal cancer. However, no such mutations in Japanese and North European as well as from Italian patients with colorectal cancer were found[3-6].
In this research, 110 primary colorectal cancers (38 in Duke’s stage C and 25 in Duke’s stage D) and 20 liver metastases were screened by RFLP analysis. Only one sample showed positive results (Figure 2) and was confirmed by direct sequencing, while its adjacent normal intestinal mucosa and blood kept intact. Our results indicated that activating mutation of c-src might be only a rare case, which could not explain the c-src activation always found in colorectal cancer.
c-src can be activated through various mechanisms, the core of which is to relieve the intramolecular autoinhibition created by binding of its SH2 domain to pY527 in the carboxyl terminus[13]. pY527 is phosphorylated by the protein tyrosine kinase, Csk. Recently, Cam et al[14], found that Csk protein and its kinase activity were reduced in colorectal carcinoma and correlated with c-src kinase activity. Furthermore, Csk over-expression in mouse NL-17 cells (highly metastatic clone of mouse colon adenocarcinoma) resulted in significant suppression of metastasis, but did not affect its tumorigenicity[15], demonstrating its role in metastatic inhibition. More recently, Rengifo-Cam et al[16], found that overexpression of wild-type Csk increased cell-cell contacts, mediated by E-cadherin, could decrease the number of focal contacts and cell adhesion/migration and in vitro invasiveness. It is, therefore, worthwhile to detect the activity of Csk in colorectal cancer. However, Be抧istant et al[17], reported the existence of autoantibodies to Csk and elevated Csk in colorectal adenocarcinoma. Furthermore, it was found that src was deregulated in all of the tumors tested, suggesting that Csk could not phosphorylate src in transformed cells or that src was activated despite its phosph-orylation by Csk.
Apart from down-regulation of csk, transcriptional activation might be another mechanism of c-src activation in advanced colorectal cancer[18]. Our study demonstrates that activating point mutation in codon 531 may not be the main mechanism of c-src activation in colorectal cancer.
| 1. | Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 906] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 2. | Irby RB, Mao W, Coppola D, Kang J, Loubeau JM, Trudeau W, Karl R, Fujita DJ, Jove R, Yeatman TJ. Activating SRC mutation in a subset of advanced human colon cancers. Nat Genet. 1999;21:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 359] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 3. | Daigo Y, Furukawa Y, Kawasoe T, Ishiguro H, Fujita M, Sugai S, Nakamori S, Liefers GJ, Tollenaar RA, van de Velde CJ. Absence of genetic alteration at codon 531 of the human c-src gene in 479 advanced colorectal cancers from Japanese and Caucasian patients. Cancer Res. 1999;59:4222-4224. [PubMed] |
| 4. | Nilbert M, Fernebro E. Lack of activating c-SRC mutations at codon 531 in rectal cancer. Cancer Genet Cytogenet. 2000;121:94-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Wang NM, Yeh KT, Tsai CH, Chen SJ, Chang JG. No evidence of correlation between mutation at codon 531 of src and the risk of colon cancer in Chinese. Cancer Lett. 2000;150:201-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Laghi L, Bianchi P, Orbetegli O, Gennari L, Roncalli M, Malesci A. Lack of mutation at codon 531 of SRC in advanced colorectal cancers from Italian patients. Br J Cancer. 2001;84:196-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Blin N, Stafford DW. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976;3:2303-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1899] [Cited by in RCA: 2284] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 8. | Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5970] [Cited by in RCA: 6345] [Article Influence: 176.3] [Reference Citation Analysis (0)] |
| 9. | Orita M, Suzuki Y, Sekiya T, Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989;5:874-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2332] [Cited by in RCA: 2296] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 10. | Warmuth M, Damoiseaux R, Liu Y, Fabbro D, Gray N. SRC family kinases: potential targets for the treatment of human cancer and leukemia. Curr Pharm Des. 2003;9:2043-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Talamonti MS, Roh MS, Curley SA, Gallick GE. Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J Clin Invest. 1993;91:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 315] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Iravani S, Mao W, Fu L, Karl R, Yeatman T, Jove R, Coppola D. Elevated c-Src protein expression is an early event in colonic neoplasia. Lab Invest. 1998;78:365-371. [PubMed] |
| 13. | Schlessinger J. New roles for Src kinases in control of cell survival and angiogenesis. Cell. 2000;100:293-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 231] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 14. | Cam WR, Masaki T, Shiratori Y, Kato N, Ikenoue T, Okamoto M, Igarashi K, Sano T, Omata M. Reduced C-terminal Src kinase activity is correlated inversely with pp60(c-src) activity in colorectal carcinoma. Cancer. 2001;92:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Nakagawa T, Tanaka S, Suzuki H, Takayanagi H, Miyazaki T, Nakamura K, Tsuruo T. Overexpression of the csk gene suppresses tumor metastasis in vivo. Int J Cancer. 2000;88:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Rengifo-Cam W, Konishi A, Morishita N, Matsuoka H, Yamori T, Nada S, Okada M. Csk defines the ability of integrin-mediated cell adhesion and migration in human colon cancer cells: implication for a potential role in cancer metastasis. Oncogene. 2004;23:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Bénistant C, Bourgaux JF, Chapuis H, Mottet N, Roche S, Bali JP. The COOH-terminal Src kinase Csk is a tumor antigen in human carcinoma. Cancer Res. 2001;61:1415-1420. [PubMed] |
| 18. | Dehm S, Senger MA, Bonham K. SRC transcriptional activation in a subset of human colon cancer cell lines. FEBS Lett. 2001;487:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |