Published online Apr 21, 2005. doi: 10.3748/wjg.v11.i15.2340
Revised: May 26, 2004
Accepted: May 30, 2004
Published online: April 21, 2005
AIM: Oxygen free radical mediated tissue damage is well established in pathogenesis of acute pancreatitis (AP). Whether nitric oxide (NO) plays a deleterious or a protective role is unknown. In alcohol-induced AP, we studied NO, lipooxidative damage and glutathione in pancreas, lung and circulation.
METHODS: AP was induced in rats (n = 25) by injection of ethyl alcohol into the common biliary duct. A sham laparatomy was performed in controls (n = 15). After 24 h the animals were killed, blood and tissue sampling were done.
RESULTS: Histopathologic evidence confirmed the development of AP. Marked changes were observed in the pulmonary tissue. Compared with controls, the AP group displayed higher values for NO metabolites in pancreas and lungs, and thiobarbituric acid reactive substances in circulation. Glutathione was lower in pancreas and in circulation. Glutathione and NO were positively correlated in pancreas and lungs of controls but negatively correlated in circulation of experimental group. In the experimental group, plasma thiobarbituric acid reactive substances were negatively correlated with pancreas thiobarbituric acid reactive substances but positively correlated with pancreas NO.
CONCLUSION: NO increases in both pancreas and lungs in AP and NO contributes to the pathogenesis of AP under oxidative stress.
- Citation: Andican G, Gelisgen R, Unal E, Tortum OB, Dervisoglu S, Karahasanoglu T, Burçak G. Oxidative stress and nitric oxide in rats with alcohol-induced acute pancreatitis. World J Gastroenterol 2005; 11(15): 2340-2345
- URL: https://www.wjgnet.com/1007-9327/full/v11/i15/2340.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i15.2340
The etiological basis of acute pancreatitis (AP) is multifactorial. However, as in other inflammatory diseases, a final common pathway mediated by reactive oxygen species (ROS) appears to play a role in the associated tissue destruction both in the initiation and progression of AP[1-4]. Augmented production of ROS in a self-perpetuating manner, in excess of antioxidant defenses, occurs predominantly in activated neutrophils. Once produced, ROS could trigger various inflammatory processes. They can directly attack the lipoid matrix of biological membranes, stimulate arachidonic acid metabolism with increased production of prostaglandins, thromboxane, and leukotrienes, thereby enhancing the accumulation and adherence of neutrophils and platelets to the capillary wall[3]. Thus ROS could impair the microcirculation and disturb the microvascular integrity, resulting in decreased perfusion, increased capillary permeability and fluid transudation. Neutrophils infiltrating the pancreas have also been very recently demonstrated to contribute, via ROS, to the pathologic activation of digestive enzymes in acinar cells[1].
Nitric oxide (NO) has also been implicated in the pathogenesis of AP. NO, being a reactive free radical, contributes to the cytotoxicity of neutrophils and macrophages in the inflammatory response. Moreover, under circumstances of oxidative stress, the interaction of NO with superoxide radicals gives rise to peroxynitrite which can cause platelet aggregation, disseminated intravascular coagulation, lipid peroxidation and ubiquitous cell damage[5,6]. On the other hand, NO induces vasodilatation and prevents endothelial damage by inhibiting platelet aggregation and leukocyte adherence. Due to improvement in both pancreatic microcirculation and capillary organ perfusion throughout the body, NO has also been suggested to have a protective role in AP[7-9]. Thus the role of NO in the pathogenesis of AP remains controversial. Accordingly both inhibitors of NOS and NO donors are suggested to be protective in AP[7-11].
AP is known to be often complicated by multiple organ failure. In particular, acute lung injury occurs at an early stage of pancreatitis and significantly contributes to morbidity and mortality of this disease[12,13]. The etiology of this extrapancreatic disease has not yet been clearly elucidated. The injury in lung and the resulting adult respiratory distress syndrome have been attributed to the release of pancreas-derived proteolytic enzymes into the circulation[14]. There is also evidence of neutrophil related, ROS-mediated microvascular injury. The release of ROS from activated alveolar macrophages (AMs) has also been shown to lead to the progression of lung injury[15]. AM-derived NO was also reported to contribute to lung injury[16].
As alcoholism is the most common etiology in humans and the mechanism of injury remains unknown, we focused our attention on alcohol-induced AP. Only a few studies have been noted on alcohol-induced AP and in most of these, alcohol has not been used alone but in combination with another agent[17-19]. With regard to alcohol usage, an emerging concept is that a single episode of binge drinking may be sufficient to induce an episode of AP[20]. However, the previous dogma was that consistent ingestion of alcohol for a prolonged period was required to prime the pancreas before an episode of pancreatitis.
Thus in this experimental study we aimed to induce AP with a single high dose of alcohol and to assess NO production (stable metabolites of NO, nitrite plus nitrate; NOx)[21] and oxidative stress as reflected by lipid peroxidation and glutathione depletion in the pancreas, lungs and systemic circulation as far as our literature survey was concerned. In an alcohol-induced AP model these assessments were simultaneously made for the first time.
The study was carried out on 40 young adult male (200-320 g) Wistar Albino rats. The rats, cared for in accordance with the Guide for the Care and Use of Laboratory Animals[22], were permitted ad libitum access to standard laboratory chow (20-30 g/rat/d) and tap water for 10 d prior to experimental procedures.
The rats were divided randomly into two groups. The experimental group consisted of 25 rats and control group of 15 rats. Animals were anesthetized with ether to undergo a midline laparotomy and the duodenal portion of the pancreas was exposed. AP was induced by injection of ethyl alcohol (48%, 1 mL) into the common biliary duct. A sham laparotomy was performed in the control group. After 24 h, heparinized blood samples were taken with intracardiac puncture and rats were killed by decapitation under ether anesthesia. Lung and pancreatic tissues were obtained peroperatively and immediately frozen at -80 °C for biochemical measurements and fixed in 10% buffered formalin, embedded in paraffin for standard histologic examinations.
Thiobarbituric acid-reactive substances (TBARS), glutathione (GSH) and NO metabolites were measured in the blood and tissue samples. Tissues prepared in cold potassium phosphate buffer 100 g/L were homogenized using a Potter homogenizer. The homogenates were centrifuged at 3000 r/min for 15 min and the supernatant was used for the biochemical measurements.
Lipid peroxidation was determined by measuring the TBARS by the modified method of Buege et al[23] in the plasma and by the method of Okhawa et al[24] in tissue using 1.56×105 mol/L/cm as molar extinction coefficient. TBARS concentration in the supernatant of the tissue homogenates was expressed as µmol/g protein.
GSH concentration in erythrocytes and supernatants of the tissue homogenates was determined according to the method of Buetler et al[25] using metaphosphoric acid for protein precipitation and 5’5’-dithiobis-2-nitrobenzoic acid for color development. Erythrocyte GSH concentration was expressed as mg/g Hb using 1.36×104 mol/L/cm as molar absorption coefficient. Hemoglobin concentration was determined by the cyanomethemoglobin method[26]. GSH concentration in supernatants of the tissue homogenates was expressed as mg/g protein.
The stable metabolites of NO, nitrate (NO3-) and nitrite (NO2-) were measured as an index of NO production. NO colorimetric assay kit (Roche-Boehringer Mannheim) was used. Nitrate was first reduced to nitrite by NADPH in presence of nitrate reductase. Nitrite was reacted with sulfanilamide and naphthyl-ethylenediamine dihydrochloride to give a red-diazo dye. The diazo dye was measured spectrophotometrically at 550 nm. The concentration of NO metabolites was determined by comparison with a standard curve, which was constructed using a set of serial dilutions of nitrate.
Protein concentration was determined according to the method of Lowry (Sigma-Aldrich, Germany).
For histologic examination, the samples were cut into 4-μm thick sections and stained with hematoxylin and eosin. All slides were evaluated ‘’blindly’’ by two independent histopathologists for the presence of edema, hyperemia, infiltration by inflammatory cells, hemorrhage and necrosis.
All data were presented as mean±SD. Student’s t test was performed to evaluate significant differences between the groups. Correlation between different variables was studied by Pearson’s correlation coefficient. P<0.05 was considered statistically significant.
Histopathologic study of the pancreas in the experimental group showed hyperemia, interstitial edema, hemorrhage, inflammatory infiltration of neutrophils and mononuclear cells and focal necrotic areas. Marked changes were also observed in pulmonary histology, with hyperemia and focal atelectasic areas, peribronchial mononuclear and neutrophilic infiltration, early neutrophilic exudation in some alveoli. Some of the cases revealed abscess formation in lung parenchyma. These findings were considered as the evidence of an established AP. They were summarized as semiquantitatively analyzed results in control and AP group (Table 1).
| Pancreas | Lung | ||||||||
| Edema | Hemorrhage | Hyperemia | Necrosis | PMNinfiltration | PMN and lymphocytic infiltration | Alveolar neutrophilic exudation | Hyperemia | Focal atelectasia | |
| Control | +/- | - | + | - | - | - | - | +/- | - |
| AP | + | + | + | ++ | + | + | + | + | + |
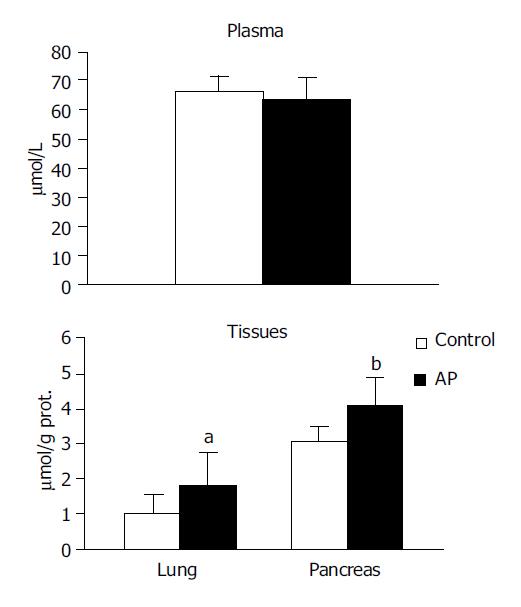
TBARS concentration in the systemic circulation was observed to be higher in the experimental group in comparison to the control (8.992±3.52 vs 6.208±0.95, P<0.05). However no significant differences were noted between the groups in pancreas or in lung (Figure 1).
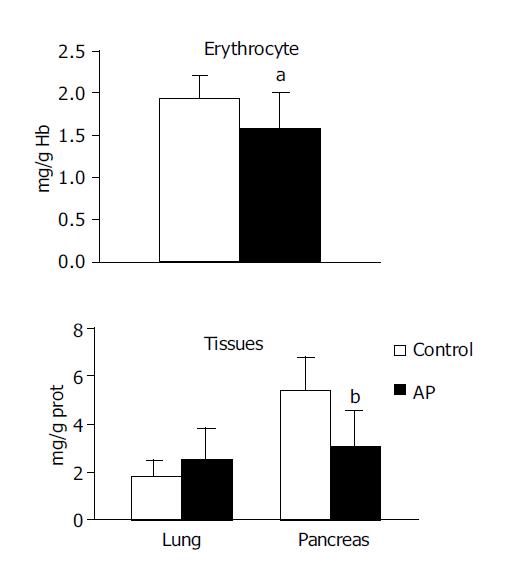
GSH concentration was observed to be significantly lower in the pancreas (2.978±1.52 vs 5.375±1.38, P<0.001) and erythrocytes (1.576±0.44 vs 1.947±0.27, P<0.05) in the experimental group compared with the control group. No significant difference was noted in GSH concentration in the lung (Figure 2).
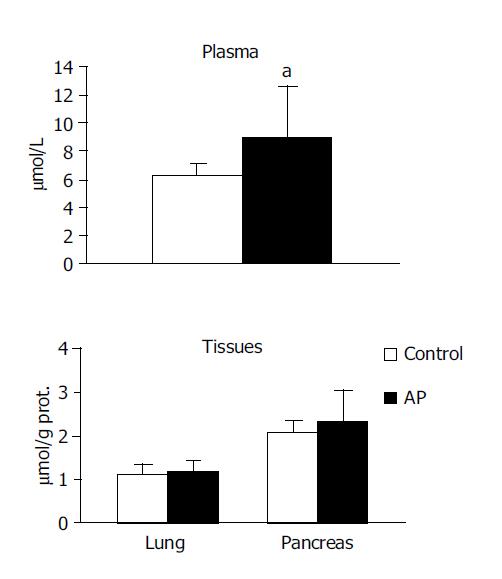
NOx were observed to be higher in both the pancreas (4.079±0.78 vs 3.080±0.37, P<0.01) and in the lung (1.809±0.89 vs 1.023±0.55, P<0.05) in the experimental group compared with the control group. No significant difference was noted in the circulation (Figure 3).
The data evaluated by correlation analysis yielded the following results (Table 2). In the control group, GSH and NO were positively correlated both in the pancreas and in the lung (r = 0.757, P<0.001; r = 0.533, P<0.05, respectively).
In the experimental group, GSH and NOx were negatively correlated in the circulation (r = -0.426, P<0.05). GSH and TBARS were positively correlated in the pancreas (r = 0.462, P<0.05). Plasma TBARS was negatively correlated with pancreas TBARS (r = -0.402, P<0.05) but positively correlated with pancreas NO (r = 0.654, P<0.01). Pancreas TBARS was positively correlated with lung TBARS (r = 0.650, P<0.01).
The pathophysiology of alcohol-related AP is not well understood. The loss in pancreatic duct integrity and/or changes in duct permeability are responsible for the initiation. Elevated intraductal pressure due to increased secretion and/or the sphincter of Oddi spasm and the presence of alcohol are considered necessary for the production of permeable ducts[17,27].
As in experimental animals not adapted to chronic alcohol ingestion ethanol given orally or intravenously had only a weak stimulatory effect on the pancreas[27], we administered alcohol to the common bile duct. Our histopathologic examination showed interstitial edema, infiltration of neutrophils and focal necrotic and hemorrhagic areas in the pancreas 24 h after the administration of alcohol. We considered these findings as lines of evidence of mild-moderate, edematous pancreatitis. Marked changes were also observed in pulmonary tissue with hyperemia, focal atelectasia, peribronchial mononuclear and neutrophilic infiltration and alveolar neutrophilic exudation resulting in abscess formation in some of the rats with pancreatitis.
High dose of ethanol is known to affect the lipids of cellular membranes and to cause alterations in membranes, permeability and functioning of the integral membrane components[18]. The loss in lysosomal membrane integrity within acinar cells and decompartmentalization of cathepsin B results in pathologic activation of trypsinogen, chymotrypsinogen, proelastase and thus autodigestion of pancreas. Additionally, ethanol affects the antiproteinase components and disturbs the proteinase-antiproteinase balance in pancreatic juice, enabling more effective activation of proteolytic enzymes. Trypsin induced activation of the complement system could contribute to inflammatory interstitial infiltration, parenchymal necrosis, and vacuolization of acinar cells. Activation of proelastase could contribute to damage in microvasculature[19]. There is also growing evidence that ethanol exerts direct toxic effects on pancreatic acinar cells. Certain toxic effects of ethanol have been attributed to both oxidative and nonoxidative metabolites of alcohol, acetaldehyde and fatty acid ethyl ester, respectively[18,28].
As to the pathogenesis of lung injury secondary to AP, it is complex and probably involves pancreatic proteases, phospholipase A2, activated complements, AMs and neutrophils[16,29]. Ethanol could specifically amplify the effect of phospholipase A2 which damages pulmonary surfactants, induces AMs to release NO and promotes PAF or eicosanoid production[19].
ROS have been implicated as an important factor in both the initiation and progression of AP in all experimental models. Augmented ROS production could cause lipid peroxidation which induces either apoptosis or necrosis depending on its extent. It has been shown that a short-term, high peak radical attack is more injurious to pancreatic acinar cells than a long lasting, low-level ROS generating system[10]. In our study, under the conditions of neutrophilic infiltration and necrosis in the pancreas, respiratory burst of polymorphonuclear leukocytes, derangement in oxidative phosphorylation, activations of NADPH oxidase, myeloperoxidase, xanthine oxidase and ethanol-catabolizing oxidases all contributed to augmented ROS production[3,30]. The increased TBARS and the decreased GSH that we observed in the circulation of rats 24 h after establishment of alcohol-induced AP reflected oxidative stress on whole body basis. As sham-operated animals served as controls these findings did not reflect laparotomy or bowel-manipulation induced alterations.
Despite an increase of lipid peroxidation in the circulation, we could not demonstrate any increase of lipid peroxidation in tissue samples. The negative correlation of TBARS in pancreatic tissue, with TBARS in circulation suggested the release of lipooxidative damage end-products from the tissue to the circulation. This release might well be due to the autodigestion in the pancreatic tissue. On the other hand, it is also known that injury to the pancreatic tissue during AP was followed by a spontaneous reparative/regenerative process. Accordingly, in several experimental studies, lipid peroxidation, observed to be increased as early as 30 min after induction of pancreatitis was normalized within 12 h[10,31]. Thus, in this respect, lipid peroxidation may perhaps be considered as the sequel of pancreatic inflammation and TBARS in circulation may likely reflect the severity of the systemic inflammatory response, rather than the pancreatic parenchymal damage. Accordingly various reactive aldehydes reflected by TBARS are rather long lived and therefore can diffuse from their site of origin and attack distant targets with some oxidative stress associated pathophysiologic effects. Interestingly we observed that the extent of lipooxidative damage in the lung was correlated with that in the pancreas.
Consumption of GSH as an antioxidant in inflammation and/or its cleavage by the activated carboxypeptidase might account for GSH depletion that we observed in rats with AP. GSH depletion might in turn cause damage to the mitochondria resulting in both ATP loss and increased ROS formation[32,33]. Additionally and importantly, GSH has also roles in acinar stimulus-secretion coupling, maintenance of the cytoskeleton and appropriate protein folding in the endoplasmic reticulum. Thus GSH depletion may also contribute to impaired zymogen granule transport (secretory block) and premature activation of pancreatic proenzymes[34].
Increased lipid peroxidation and depletion of intracellular GSH in pancreatic tissue in alcohol-induced AP have been reported[17,35,36]. However, it was difficult to compare these data due to the differences in the experimental models and the varying time points of measurements.
Our findings of positively correlated GSH and TBARS in the pancreatic tissues of rats with AP may suggest that depletion of GSH and removal of lipid peroxides occur simultaneously. It may as well be suggested that these are different aspects of the same autodigestive process. As discussed above, lipid peroxide removal might be due to two opposing processes, the detrimental autodigestion and the repair. However, GSH depletion irrespective of the mechanism, represents cellular deterioration and progression of the pathologic process. Relative to earlier repair in the lipoid matrix, long lasting GSH depletion has been reported[10].
In the AP group we observed increased NO production in both of the involved organs, pancreas and lung in comparison to the control. Under prevailing conditions of inflammation and oxidative stress NO became damaging to the tissue, specifically to the endothelium. Specific immunohistochemical staining revealed that the lung and pancreas were important targets of oxidative stress caused by peroxynitrite in AP. The subsequent loss of NO bioactivity further contributed to the endothelial dysfunction[5]. Accordingly, we observed that increased NO production in pancreas was correlated with increased circulatory lipid peroxides in alcohol-induced AP group. The negative correlation of NO and GSH in the circulation of this group suggested that increased NO production was related with GSH depletion in alcohol-induced AP. In accordance to our findings, NO has been reported to compromise the cellular redox state via oxidation of thiols like GSH[10]. In contrast to the AP group, we observed a positive correlation between GSH and NO in both the pancreas and lung in the control group. NO appeared to play an antioxidant role in control rats whereas as a pro-oxidant in alcohol-induced AP rats. These findings clearly indicate that whether NO behaves as an antioxidant or as a pro-oxidant is dependent on the prevailing conditions of oxidative stress[10].
Studies were not in consensus as to whether NO was cytotoxic or cytoprotective in AP[7-11]. Our findings and those reported are in accordance with the well established dual nature of NO. It may act both as a cytotoxic agent and a cytoprotective agent, the main determinants being its concentration and the environment. The action of NO during inflammatory reactions has to be also considered in the context of timing Kröncke et al[37].
In conclusion, this experimental study provides biochemical evidence for the cytotoxic role of NO under circumstances of oxidative stress in the pathophysiology of alcohol-induced pancreatitis. Further studies investigating whether modulation of oxidative stress by antioxidant therapy attenuates the cytotoxicity of NO are needed.
| 1. | Gough DB, Boyle B, Joyce WP, Delaney CP, McGeeney KF, Gorey TF, Fitzpatrick JM. Free radical inhibition and serial chemiluminescence in evolving experimental pancreatitis. Br J Surg. 1990;77:1256-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Cuzzocrea S, Mazzon E, Dugo L, Serraino I, Centorrino T, Ciccolo A, Van de Loo FA, Britti D, Caputi AP, Thiemermann C. Inducible nitric oxide synthase-deficient mice exhibit resistance to the acute pancreatitis induced by cerulein. Shock. 2002;17:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Tsai K, Wang SS, Chen TS, Kong CW, Chang FY, Lee SD, Lu FJ. Oxidative stress: an important phenomenon with pathogenetic significance in the progression of acute pancreatitis. Gut. 1998;42:850-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Sanfey H, Bulkley GB, Cameron JL. The role of oxygen-derived free radicals in the pathogenesis of acute pancreatitis. Ann Surg. 1984;200:405-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 210] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Viola G, al-Mufti RA, Sohail M, Williamson RC, Mathie RT. Nitric oxide induction in a rat model of selective pancreatic ischemia and reperfusion. Hepatogastroenterology. 2000;47:1250-1255. [PubMed] |
| 6. | Rodeberg DA, Chaet MS, Bass RC, Arkovitz MS, Garcia VF. Nitric oxide: an overview. Am J Surg. 1995;170:292-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Liu X, Nakano I, Yamaguchi H, Ito T, Goto M, Koyanagi S, Kinjoh M, Nawata H. Protective effect of nitric oxide on development of acute pancreatitis in rats. Dig Dis Sci. 1995;40:2162-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Werner J, Fernández-del Castillo C, Rivera JA, Kollias N, Lewandrowski KB, Rattner DW, Warshaw AL. On the protective mechanisms of nitric oxide in acute pancreatitis. Gut. 1998;43:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Andrzejewska A, Jurkowska G. Nitric oxide protects the ultrastructure of pancreatic acinar cells in the course of caerulein-induced acute pancreatitis. Int J Exp Pathol. 1999;80:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Schulz HU, Niederau C, Klonowski-Stumpe H, Halangk W, Luthen R, Lippert H. Oxidative stress in acute pancreatitis. Hepatogastroenterology. 1999;46:2736-2750. [PubMed] |
| 11. | Sweiry JH, Mann GE. Role of oxidative stress in the pathogenesis of acute pancreatitis. Scand J Gastroenterol Suppl. 1996;219:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Tsukahara Y, Horita Y, Anan K, Morisaki T, Tanaka M, Torisu M. Role of nitric oxide derived from alveolar macrophages in the early phase of acute pancreatitis. J Surg Res. 1996;66:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Renner IG, Savage WT, Pantoja JL, Renner VJ. Death due to acute pancreatitis. A retrospective analysis of 405 autopsy cases. Dig Dis Sci. 1985;30:1005-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 304] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | O'Donovan DA, Kelly CJ, Abdih H, Bouchier-Hayes D, Watson RW, Redmond HP, Burke PE, Bouchier-Hayes DA. Role of nitric oxide in lung injury associated with experimental acute pancreatitis. Br J Surg. 1995;82:1122-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Guice KS, Oldham KT, Caty MG, Johnson KJ, Ward PA. Neutrophil-dependent, oxygen-radical mediated lung injury associated with acute pancreatitis. Ann Surg. 1989;210:740-747. [PubMed] |
| 16. | Closa D, Sabater L, Fernández-Cruz L, Prats N, Gelpí E, Roselló-Catafau J. Activation of alveolar macrophages in lung injury associated with experimental acute pancreatitis is mediated by the liver. Ann Surg. 1999;229:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Lüthen RE, Niederau C, Grendell JH. Glutathione and ATP levels, subcellular distribution of enzymes, and permeability of duct system in rabbit pancreas following intravenous administration of alcohol and cerulein. Dig Dis Sci. 1994;39:871-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Rydzewska G, Jurkowska G, Dziecioł J, Faszczewska A, Wróblewski E, Gabryelewicz A. Does chronic ethanol administration have influence on pancreatic regeneration in the course of caerulein induced acute pancreatitis in rats. J Physiol Pharmacol. 2001;52:835-849. [PubMed] |
| 19. | Dlugosz JW, Wroblewski E, Poplawski C, Gabryelewicz A, Andrzejewska A. Does antecedent ethanol intake affect course of taurocholate pancreatitis in rats? Dig Dis Sci. 1997;42:944-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Somogyi L, Martin SP, Venkatesan T, Ulrich CD. Recurrent acute pancreatitis: an algorithmic approach to identification and elimination of inciting factors. Gastroenterology. 2001;120:708-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Upchurch GR, Welch GN, Fabian AJ, Freedman JE, Johnson JL, Keaney JF, Loscalzo J. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem. 1997;272:17012-17017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 488] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 22. | Guide for the care and use of laboratory animals: Committee on care and use of laboratory animals. Institute of laboratory animal resources, National Research Council 1995: 83. . |
| 23. | Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7899] [Cited by in RCA: 7982] [Article Influence: 169.8] [Reference Citation Analysis (0)] |
| 24. | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17627] [Cited by in RCA: 18806] [Article Influence: 408.8] [Reference Citation Analysis (0)] |
| 25. | Buetler E, Duran O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;51:882-888. |
| 26. | Fairbanks V, Klee GG. Biochemical aspects of hematology. In Tietz NW, eds. Textbook of Clinical Chemistry. Philadelphia: WB Saunders Company 1986; 1532-1534. |
| 27. | Harvey MH, Cates MC, Reber HA. Possible mechanisms of acute pancreatitis induced by ethanol. Am J Surg. 1988;155:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Werner J, Laposata M, Fernández-del Castillo C, Saghir M, Iozzo RV, Lewandrowski KB, Warshaw AL. Pancreatic injury in rats induced by fatty acid ethyl ester, a nonoxidative metabolite of alcohol. Gastroenterology. 1997;113:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 140] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Tsukahara Y, Morisaki T, Horita Y, Torisu M, Tanaka M. Phospholipase A2 mediates nitric oxide production by alveolar macrophages and acute lung injury in pancreatitis. Ann Surg. 1999;229:385-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Schoenberg MH, Büchler M, Beger HG. Oxygen radicals in experimental acute pancreatitis. Hepatogastroenterology. 1994;41:313-319. [PubMed] |
| 31. | Dabrowski A, Chwiećko M. Oxygen radicals mediate depletion of pancreatic sulfhydryl compounds in rats with cerulein-induced acute pancreatitis. Digestion. 1990;47:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Schoenberg MH, Büchler M, Younes M, Kirchmayr R, Brückner UB, Beger HG. Effect of antioxidant treatment in rats with acute hemorrhagic pancreatitis. Dig Dis Sci. 1994;39:1034-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Kruse P, Anderson ME, Loft S. Minor role of oxidative stress during intermediate phase of acute pancreatitis in rats. Free Radic Biol Med. 2001;30:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Gómez-Cambronero L, Camps B, de La Asunción JG, Cerdá M, Pellín A, Pallardó FV, Calvete J, Sweiry JH, Mann GE, Viña J. Pentoxifylline ameliorates cerulein-induced pancreatitis in rats: role of glutathione and nitric oxide. J Pharmacol Exp Ther. 2000;293:670-676. [PubMed] |
| 35. | Ozaras R, Tahan V, Aydin S, Uzun H, Kaya S, Senturk H. N-acetylcysteine attenuates alcohol-induced oxidative stress in the rat. World J Gastroenterol. 2003;9:125-128. [PubMed] |
| 36. | Wittel UA, Bachem M, Siech M. Oxygen radical production precedes alcohol-induced acute pancreatitis in rats. Pancreas. 2003;26:e74-e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Kröncke KD, Suschek CV, Kolb-Bachofen V. Implications of inducible nitric oxide synthase expression and enzyme activity. Antioxid Redox Signal. 2000;2:585-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |