Published online Apr 14, 2005. doi: 10.3748/wjg.v11.i14.2203
Revised: October 24, 2004
Accepted: November 29, 2004
Published online: April 14, 2005
Extraskeletal myxoid chondrosarcoma (EMC) is a low-grade sarcoma characterized by developing metastases and local recurrence in high rate. It is mainly deep seated in the proximal extremities. The most common metastatic sites are the lungs, soft tissues, lymph nodes, bones and the brain. To our knowledge, no case of clearly defined EMC has been reported to date developing a metastasis in the pancreas. We describe a case of a man suffering from EMC who developed a single pancreatic metastasis 20 years after the initial diagnosis. A 49-year-old man was submitted to surgical excision of an EMC, in left thigh, 20 years ago. Fourteen years after the initial diagnosis a local recurrence in left thigh occurred. Multiple lesions of metastatic origin, in both lungs, were excised via thoracotomies until the time being. In 2003, as a part of a periodically performed imaging control, an abdominal CT scan was performed revealing a solid lesion in the pancreas. Distal pancreatectomy was performed. The histopathology of the excised specimen proved to be the one of metastatic lesion of EMC. The above-mentioned case of EMC is, as far as we know, the first one described developing a certain pancreatic metastasis.
- Citation: Fotiadis C, Charalambopoulos A, Chatzikokolis S, Zografos G, Genetzakis M, Tringidou R. Extraskeletal myxoid chondrosarcoma metastatic to the pancreas: A case report. World J Gastroenterol 2005; 11(14): 2203-2205
- URL: https://www.wjgnet.com/1007-9327/full/v11/i14/2203.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i14.2203
Extraskeletal myxoid chondrosarcoma (EMC) was described firstly by Stout and Verner[1] in 1953 and as a distinct clinicopathologic entity by Enziger and Shiraki[2] in 1972. It is generally viewed as a low-grade sarcoma composed of primitive chondroid cells in an abundant myxoid matrix resembling embryonic cartilage and presenting a high rate of survival. However, despite the protracted clinical course, it is characterized by developing metastases and local recurrence in high rate and finally high mortality[3].
EMC represents 2.5% of soft tissue sarcomas and the male to female ratio is 2:1. At the time of diagnosis, the median tumor size ranges from 1 to 25 cm in diameter, while the patient’s median age is 50 years (ranging from 6 to 90 years)[1,4-6]. EMC is mainly deep seated in the proximal extremities, most frequently presenting as a painful soft tissue mass. Almost half of the cases will recur during the clinical course[4,6].
About half of the cases of EMC will develop metastases, which is proved to be an adverse prognostic factor with regard to survival[4]. The location of metastases depends on the primary site of the lesion. The most common metastatic sites[4] are the lungs, soft tissues, lymph nodes, bone[7] and the brain. Rare cases of metastatic EMC are noted in the liver[8], the mandibular[9], the retroperitoneum[10] and the right ventricle[11].
The detection of metastases varies from 6 to 15 years after the initial diagnosis of the tumor, while survival after detection ranges from a few months to 18 years[4]. Metastases are detected before identification of the primary lesion or at the time of diagnosis in almost one-third of cases developing metastases. Significant adverse prognostic factors, with regard to metastases, are larger tumor size, microscopic tumor necrosis and higher Ki67 values[4].
We describe a case of a man suffering from EMC who developed a single pancreatic metastasis 20 years after the initial diagnosis. To our knowledge, no case of clearly defined EMC has been reported to date developing a metastasis in the pancreas.
A 49-year-old man was submitted to surgical excision of a soft tissue mass of unknown origin, in left thigh, 20 years ago. The histopathologic diagnosis of the surgical specimen, 2.5 cm in diameter, was referring to myxoid liposarcoma of the thigh. Fourteen years after the initial diagnosis, in 1997, a local recurrence in left thigh occurred, viewed in MRI. The lesion, 3 cm×2 cm×2 cm in dimensions, was being excised and the histopathologic findings showed EMC. Moreover, a piece of left femur 1.5 cm×1.5 cm×1 cm also excised, proved bone superficially permeated by the lesion. A review of the histological slides of the primary tumor also revealed EMC.
In 2000, an imaging control was performed because of developing the local recurrence three years ago. Multiple lesions of metastatic origin in both lungs were found. The patient was submitted to thoracotomy and four lesions, ranging in diameter from 0.6 to 3 cm, were excised from both lungs. The lesions proved to be metastatic of EMC. During recent three years, three surgical excisions of metastatic lesions in both lungs took place: thoracotomy in 2002 for two lesions and two thoracotomies in 2003.
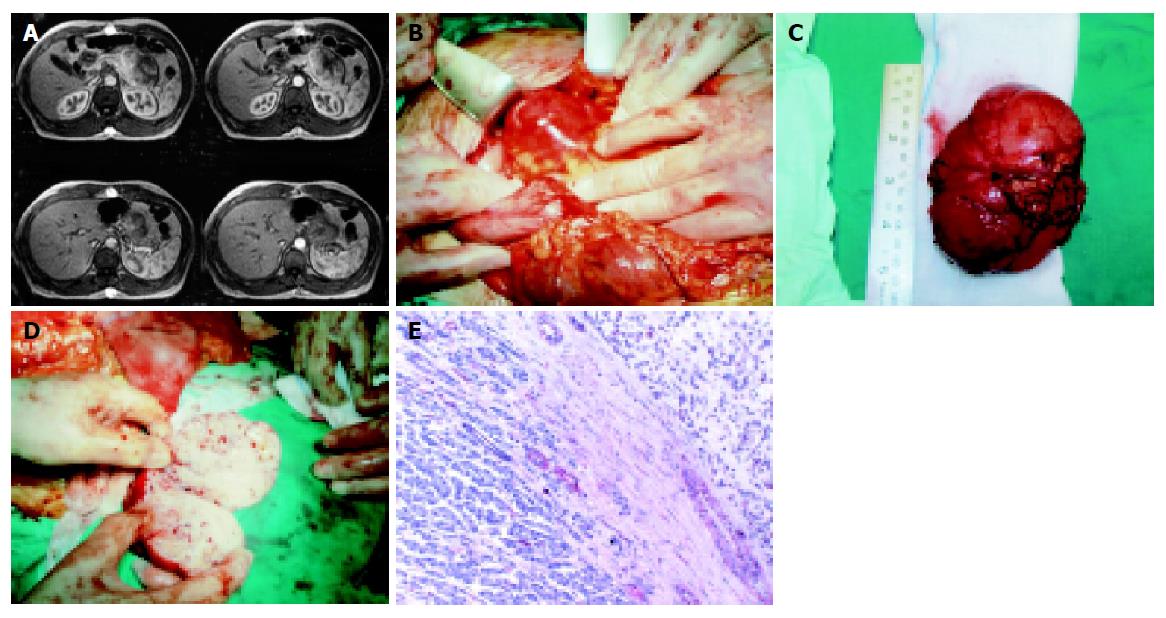
In May of 2003, as a part of a periodically performed imaging control, an abdominal CT scan initially as well as an MRI were performed. These studies revealed a solid, in morphology, lesion with distinct margins, about 9 cm × 6 cm in dimensions (Figure 1A). This lesion was viewed just anterior to the body and the tail of the pancreas, giving the impression to be of pancreatic origin. The clinical and imaging features of the lesion were claiming to be of metastatic nature from EMC. As a part of the diagnosis process, a biopsy of the tumor after CT-guided FNA was performed, but the material of aspiration was not of any diagnostic significance.
Laparotomy was decided, firstly to determine the nature of the tumor and secondly for therapeutical reasons, if feasible. The lesion, previously described, was discovered originating from the body and the tail of the pancreas. Distal pancreatectomy was performed (Figure 1B-1D).
The histological examination of the excised specimen proved to be the one of the metastatic lesion of EMC (Figure 1E) including tumor cells oval to round in shape, as well as some spindled ones, with light colored/eosinophilic cytoplasm. They were arranged, as single ones or in small groups, in an abundant myxoid matrix. The immunohistochemical staining results were positive in vimentin, a1-antithrypsin, a1-antichymothrypsin, lysozyme and protein S-100, while negative in all the epithelial factors.
During the most recently performed follow-up of the patient, five months after the pancreatectomy, a CT of the chest revealed a, possibly metastatic, lesion in the right medial lobe 1.2 cm in diameter. Furthermore, an abdominal CT imaged a slightly viewed lesion in the quadrate lobe of the liver, which could not be characterized, because of its dimensions, without excluding the metastatic nature.
EMC is a rare neoplasm of soft tissue located in the proximal extremities and limb girdles in 60% of cases, the trunk (20%) and the distal extremities (20%). Distal is defined as those lesions distal to the knee or elbow[1,4]. Most tumors arise within skeletal muscle or tendious structures although some involve deep subcutis[4]. Involving skin or invading bone is extremely rare.
The local recurrence rate is about 45-50% and is not a prognostic factor of survival[4]. Time passing between the initial diagnosis and the first local recurrence ranges from a few months to 18 years. With regard to local recurrence, adverse prognostic factors are female sex and higher mitotic rate[4].
The differential diagnosis of EMC is broad and depends on whether the individual case is hypercellular, hypocellular or a typical EMC. Benign and malignant tumors are included, as mixed myoepithelial tumors[12], chondroid lipoma[13], myxoid liposarcoma, myxofibrosarcoma[4], low-grade fibromyxoid sarcoma, myxoid variants of ossifying fibromyxoid tumor of soft tissues[14] and myxoid sclerosing epithelioid fibrosarcoma.
EMC has usually a multilobular configuration with an incomplete fibrous capsule, but some cases are infiltrative. Most have fibrous septa traversing the lesion and an abundant myxoid matrix. The tumor cells, primitive chondroid, are arranged in delicate intersecting strands and rings, but in some tumors forming small balls and clusters due to loss of cell’s cohesion[4]. Some cases are viewed with epithelioid and/or rhabdoid cells. Cellular areas with minimal or even absent myxoid matrix are observed in several cases, while other cases are hypocellular and composed of elongated, spindled cells reminiscent to fibroblasts and myofibroblasts, this way confusing differential diagnosis. Histologic features, including necrosis, increased cellularity, high mitotic rate, pleomorphism, epithelioid features, rhabdoid cells and spindled foci are not proven to affect prognosis[4].
A recurrent t (9:22) (q22:q12) translocation has been reported in EMC involving the EWS gene at 22q12 and a novel gene at 9q22 designated TEC or CHN, which encodes for a novel orphan nuclear receptor. Two major types of EWS-TEC fusion transcripts have been identified, accounting for 75% of EMC[15,18]. Recent findings suggest that a specific gene, TAF2N (member of TLS/FUS family, where EWS gene also belongs to), can replace EWS gene in the fusion transcript. The novel TAF2N-TEC gene fusion could account for those 25% of cases that lack[19].
The study of certain immunohistochemical features reveals some of them, as p53 and Ki67, with negative or extremely weak immunostaining, while others, as protein S-100, vimentin, PCNA, bcl-2, enolase, EMA and laminin, with variant degrees of staining, without however any prognostic role[4,5,20].
CT and MRI possess a major role during the diagnostic procedure, with the latter frequently imaging tumors of lobulated appearance with enhancement following contrast injection[4]. The role of cytogenetics in the diagnosis becomes extremely important, particularly in view of hypercellular, nonmyxoid and potentially solid variants of EMC[15-18]. The biopsy of the tumor after fine needle aspiration (FNA) has not yet been proved of significance in diagnosis[21].
The treatment of EMC is problematic because it has the propensity to recur locally and most cases do not respond well to chemotherapy[22] and radiation. Complete surgical excision, when feasible, remains the primary treatment modality for EMC, with radiation therapy and chemotherapy reserved for unique cases[4]. Specific surgical modifications could decrease the postoperative complications.
It seems that certain clinical features such as tumor’s size, its location (proximal of the extremities), patient’s age and the presence of metastases are significant adverse prognostic factors of survival, although not clearly defined[4]. The estimation of 5-, 10- and 15-year survival rates of EMC is accounted up to 90%, 70% and 60%, respectively[4].
There are two references in the literature describing pancreatic metastases, but the histopathologic character of the chondrosarcoma is not clearly defined.
Concluding, the case of EMC, mentioned above, is, as far as we know, the first one described developing a certain pancreatic metastasis.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Stout AP, Verner EW. Chondrosarcoma of the extraskeletal soft tissues. Cancer. 1953;6:581-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Enzinger FM, Shiraki M. Extraskeletal myxoid chondrosarcoma. An analysis of 34 cases. Hum Pathol. 1972;3:421-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 241] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Saleh G, Evans HL, Ro JY, Ayala AG. Extraskeletal myxoid chondrosarcoma. A clinicopathologic study of ten patients with long-term follow-up. Cancer. 1992;70:2827-2830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Meis-Kindblom JM, Bergh P, Gunterberg B, Kindblom LG. Extraskeletal myxoid chondrosarcoma: a reappraisal of its morphologic spectrum and prognostic factors based on 117 cases. Am J Surg Pathol. 1999;23:636-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 193] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Okamoto S, Hisaoka M, Ishida T, Imamura T, Kanda H, Shimajiri S, Hashimoto H. Extraskeletal myxoid chondrosarcoma: a clinicopathologic, immunohistochemical, and molecular analysis of 18 cases. Hum Pathol. 2001;32:1116-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Saleh G, Evans HL, Ro JY, Ayala AG. Extraskeletal myxoid chondrosarcoma. A clinicopathologic study of ten patients with long-term follow-up. Cancer. 1992;70:2827-2830. |
| 7. | Takeda A, Tsuchiya H, Mori Y, Nonomura A, Tomita K. Extraskeletal myxoid chondrosarcoma with multiple skeletal metastases. J Orthop Sci. 2000;5:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Ryan JM, Dupuy DE, Pitman M, Boland GW, Hahn PF, Mueller PR. Metastases to the liver from extraskeletal myxoid chondrosarcoma and successful treatment with percutaneous ethanol injection. Clin Radiol. 2000;55:314-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Englert TP, Kahn MR, Bushkoff SH, Mendelow H. Mandibular metastasis of an extraskeletal myxoid chondrosarcoma arising on the plantar surface of the foot: report of case. J Oral Surg. 1978;36:401-405. [PubMed] |
| 10. | Gebhardt MC, Parekh SG, Rosenberg AE, Rosenthal DI. EMC of the knee. Skeletal Radiol. 2000;28:354-358. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Banfić L, Jelić I, Jelasić D, CuZić S. Heart metastasis of extraskeletal myxoid chondrosarcoma. Croat Med J. 2001;42:199-202. [PubMed] |
| 12. | Kilpatrick SE, Hitchcock MG, Kraus MD, Calonje E, Fletcher CD. Mixed tumors and myoepitheliomas of soft tissue: a clinicopathologic study of 19 cases with a unifying concept. Am J Surg Pathol. 1997;21:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 195] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Meis JM, Enzinger FM. Chondroid lipoma. A unique tumor simulating liposarcoma and myxoid chondrosarcoma. Am J Surg Pathol. 1993;17:1103-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 74] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Enzinger FM, Weiss SW, Liang CY. Ossifying fibromyxoid tumor of soft parts. A clinicopathological analysis of 59 cases. Am J Surg Pathol. 1989;13:817-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 180] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Hirabayashi Y, Ishida T, Yoshida MA, Kojima T, Ebihara Y, Machinami R, Ikeuchi T. Translocation (9; 22)(q22; q12). A recurrent chromosome abnormality in extraskeletal myxoid chondrosarcoma. Cancer Genet Cytogenet. 1995;81:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Stenman G, Andersson H, Mandahl N, Meis-Kindblom JM, Kindblom LG. Translocation t(9; 22)(q22; q12) is a primary cytogenetic abnormality in extraskeletal myxoid chondrosarcoma. Int J Cancer. 1995;62:398-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Sjögren H, Wedell B, Meis-Kindblom JM, Kindblom LG, Stenman G. Fusion of the NH2-terminal domain of the basic helix-loop-helix protein TCF12 to TEC in extraskeletal myxoid chondrosarcoma with translocation t(9; 15)(q22; q21). Cancer Res. 2000;60:6832-6835. [PubMed] |
| 18. | Brody RI, Ueda T, Hamelin A, Jhanwar SC, Bridge JA, Healey JH, Huvos AG, Gerald WL, Ladanyi M. Molecular analysis of the fusion of EWS to an orphan nuclear receptor gene in extraskeletal myxoid chondrosarcoma. Am J Pathol. 1997;150:1049-1058. [PubMed] |
| 19. | Sjögren H, Meis-Kindblom J, Kindblom LG, Aman P, Stenman G. Fusion of the EWS-related gene TAF2N to TEC in extraskeletal myxoid chondrosarcoma. Cancer Res. 1999;59:5064-5067. [PubMed] |
| 20. | Oliveira AM, Sebo TJ, McGrory JE, Gaffey TA, Rock MG, Nascimento AG. Extraskeletal myxoid chondrosarcoma: a clinicopathologic, immunohistochemical, and ploidy analysis of 23 cases. Mod Pathol. 2000;13:900-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Jones H, Ozua PO, Lee DM. Diagnosis of EMC by FNA. Cytopathol. 1995;6:273-276. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Patel SR, Burgess MA, Papadopoulos NE, Linke KA, Benjamin RS. Extraskeletal myxoid chondrosarcoma. Long-term experience with chemotherapy. Am J Clin Oncol. 1995;18:161-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |