Published online Apr 14, 2005. doi: 10.3748/wjg.v11.i14.2193
Revised: September 21, 2004
Accepted: November 23, 2004
Published online: April 14, 2005
AIM: To evaluate ophthalmic disorders with special attention to retinopathy in cirrhotic patients. Vitamin A deficiency-related ophthalmopathy, xerophthalmia, and color blindness may be documented in cirrhosis due to various etiologies. Retinopathy is an obscure feature of cirrhosis.
METHODS: Thirty-two cirrhotic patients, who were followed up by Clinics of Gastroenterology, Izmir Ataturk Teaching and Research Hospital, were enrolled to the study. Associated systemic diseases such as diabetes mellitus and hypertension were excluded. Thirty-two healthy volunteers took part as the control subjects. All participants had ophthalmologic examination in the same hospital.
RESULTS: Five (15.6%) of the cirrhotic subjects had soft exudate in the retina. None of the control subjects had retinopathy (P<0.05). Intraocular pressure (IOP) measured for both eyes were also significantly lower in the cirrhotics (P<0.05 vs P = 0.01). There were no statistically significant differences between the two groups in terms of other ophthalmic pathologies. The ophthalmic findings did not show up any differences according to the etiology of cirrhosis.
CONCLUSION: Soft exudates may develop in cirrhotic patients probably due to loss of synthetic function of liver and hemodynamic effects of portal hypertension. Retinopathy must be sought in cirrhosis because of its severe morbidity.
- Citation: Onder C, Bengur T, Selcuk D, Bulent S, Belkis U, Ahmet M, Eser P, Leyla AS. Relationship between retinopathy and cirrhosis. World J Gastroenterol 2005; 11(14): 2193-2196
- URL: https://www.wjgnet.com/1007-9327/full/v11/i14/2193.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i14.2193
More than 60% of cirrhotic patients have clinical manifestations of portal hypertension (splenomegaly, portal-systemic collaterals, ascites)[1]. The ophthalmic pathologies of cirrhosis in the literature involve xerophthalmia, vitamin A deficiency, and color blindness. Xerophthalmia and keratoconjunctivitis observed in Sjögren’s syndrome may be associated with autoimmune hepatitis and primary biliary cirrhosis. Xeroph-thalmia is also observed in vitamin A deficiency. It may even progress to small grayish lesions, namely Bitot’s spots, and blindness. Night blindness may ensue. Vitamin A deficiency may be seen in cirrhosis since liver is the organ where vitamin A is deposited[2,3]. This is a well-documented state in primary biliary cirrhosis and alcoholic cirrhosis. Color blindness may be seen in cirrhosis especially in alcoholic type.
There are scarcely few studies about retinopathy in cirrhosis. Exudates and hemorrhage may be seen in the retina[4,5]. In these studies, associated diseases such as DM and hypertension were not excluded. Relationship between retinopathy and a variety of systemic diseases such as diabetes mellitus (DM), hypertension, blood dyscrasias (myeloproli-ferative disorders, plasma cell dyscrasias), Behçet’s disease, sarcoidosis and systemic infections including HIV is well known. Intraretinal hemorrhage, exudate, cotton-wool spots (retinal infarcts), and papilla edema may be seen in hyper-tensive neuroretinopathy and diabetic retinopathy. These changes constitute an important morbidity since they may lead to blindness. Exudates are composed of extravasated plasma proteins mainly lipoproteins and are gray-yellow in color. Macrophage response is also evident. Cotton-wool spots represent microvascular infarcts due to ischemia. Axoplasmic deposits surround the infarcted area. Diabetic retinopathy comprises endothelial dysfunction, advanced glycosylated end-products, increased capillary permeability, thrombocyte dysfunction, adherence of leukocytes to endothelium, arteriovenous shunts due to obstruction of vasculature with coagulum, retinal ischemia, retinal edema, and exudates. Diabetic retinopathy is primarily related to hyperglycemia though hormonal and hemodynamic factors may modify the ongoing process[6]. Focal and diffuse arteriolar narrowing, obstructed precapillary arterioles, hemorrhage, retinal edema, and hard exudates occur in hypertensive retinopathy. There is an entity that has to be considered in the differential diagnosis, the so-called Drusen anomaly. It precedes senile macular degeneration. It does not usually result in blindness. They are characterized by asymptomatic yellow deposits underneath the retinal pigmented layer[7].
Thirty-two cirrhotic patients followed up by Clinics of Gastroenterology, Izmir Ataturk Teaching and Research Hospital were enrolled to the study. All patients had clinical, laboratory, and ultrasonographic features compatible with cirrhosis. Hepatitis B, alcohol, and both causes were responsible for the cirrhotic cases (16, 14, 2 patients, respectively). None of the patients had hepatitis C infection. They were neither diabetic (according to fasting and random blood glucose) nor hypertensive according to the criteria of American Dia-betes Association and Joint National Committee[8,9]. They were not using any ophthalmic and antidiabetic drugs. The only two cardiovascular medicine used were propranolol (81%) and spironolactone and/or furosemide (63%) prescribed for cirrhosis. All patients had endoscopic exami-nation within the preceding 6 mo due to several reasons and esophageal and gastric varices were detected in 84% and 44%, respectively. The control group comprised 32 healthy volunteers. None of them had a known history of DM, hypertension, known liver disease, and alcohol consumption; they were not using any drugs related to these disorders either.
Ophthalmic examination was performed following pupil dilation with 1% tropicamide drops. Color blindness was evaluated with Ishihara test. Intraocular pressure (IOP) was measured with the applanation tonometry. Schirmer 2 test and break-up time were measured to detect xerophthalmia. Standard Whatman filter paper was used for Schirmer test. Anesthetic drop was not applied. Values above 10 mm were accepted abnormal. Break-up time was measured with biomicroscope. Values ranging from 15 to 35 s were considered normal. The protocol was approved by the local ethics committee and each patient provided written informed consent.
The whole statistical analysis was performed using the SPSS 10.0 statistical software for Windows. Qualitative ophthalmic data were compared by χ2 test and quantitative data by nonparametric tests. We compared Schirmer test results, break-up time values, and IOP measurements first by Kruskal-Wallis test and then by Mann-Whitney nonpa-rametric test. Variables with a P value less than 0.05 was considered statistically significant.
Mean age was 47.5±9.3 years for the cirrhotic group and 43.4±14.1 years for the control group. Male was the prepo-nderant gender in the cirrhotic group (n = 25, 78%); only 18 (56%) were male in the other group. Eighteen (56%) of them were Child-Pugh class B, 7 (22%) were Child-Pugh class C, and 7 (22%) were Child-Pugh class A. Serum glucose, albumin, sodium levels, and platelet counts of the cirrhotic patients were as follows: 101±11 mg/dL, 3.08±0.65 g/dL, 135±5 meq/L, and 111 567±63 687/mm3, respectively. Twenty-five patients had a mean activated partial thromboplastin time of 36.7±6.0 s. All patients had prothrombin time (PT) measured (mean 16.4±5.3 s) (Table 1).
| Cirrhotic group | Healthy group | |
| Age (yr) | 47.5±9.3 | 43.4±14.1 |
| Gender (male/female) | 25/7 (78%/22%) | 18/14 (56%/44%) |
| Glucose (mg/dL) | 101±11 | |
| Albumin (g/dL) | 3.08±0.65 | |
| Sodium (meq/L) | 135±5 | |
| Platelet count (/mm3) | 111567±63687 | |
| Gastric varices | 14 (44%) | |
| Esophageal varices | 27 (84%) | |
| Spider angioma | 13 (41%) | |
| Hepatitis B | 18 (56%) | - |
| Alcohol | 16 (50%) | - |
| β-blocker usage | 26 (81%) | - |
| Diuretic usage | 20 (63%) | - |
Results of Schirmer test performed for each eye separ-ately were similar (cirrhotics: 13.8±3.1 mm in the right vs 14.5±2.6 mm in the left; control subjects 15.0±1.7 mm in both eyes). In the cirrhotic group break-up time for tear film was 12.5±2.8 and 12.5±2.7 s. It was 12.2±2.1 and 11.9±2.2 s for the control subjects.
Two (6%) cirrhotic patients and one (3%) healthy volunteer were color blind. None of the cirrhotics, but two (6%) control subjects, had epithelial defect in cornea. Iris atrophy was evident in one (3%) cirrhotic patient and all control subjects had normal anterior chamber examination. Vision acuity was sound in 75% of cirrhotics and 28% of the control subjects. One patient from each group had glaucoma (3% of cirrhotics and 3% of control subjects). The two groups had no statistically significant differences in terms of ophthalmic features outlined above.
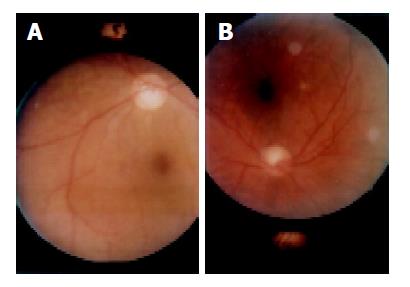
Retinal examination of cirrhotics revealed chorioretinal atrophy in two (6%) patients, Drusen anomaly in one (3%), and exudates in five (16%) (Table 2) (Figures 1A and 1B). In the latter group, exudates were localized bilaterally (n = 3, 60%). Three out of five had exudates in the macular region. One had diffuse soft exudates. None of the healthy subjects had exudates. The cirrhotic subjects had a significantly higher incidence of retinal exudates when compared to the control ones (P<0.05). All cirrhotics with retinopathy were also using diuretics. Among the cirrhotics without retinopathy 75% were treated with diuretics. However, retinopathy was not statistically relevant to diuretic usage. Ophthalmic features did not show up any significant differences neither among patients with or without gastric varices nor among those with or without spider angiomas. Etiology of cirrhosis was not shown to have any impact on ophthalmic findings. No significant differences were found between two groups in terms of Schirmer test results and break-up time values. The participants of both groups had IOP in the normal range. However, IOP was significantly lower in the cirrhotic group (P<0.05 and P = 0.01) (Table 3). Hence, we did not observe any significant difference between ophthalmic findings of both groups, and IOP values. Cirrhosis may occasionally cause hemorrhage in ophthalmic structures due to impaired production of coagulation factors and thrombocytopenia. In our study no hemorrhagic foci were found.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
| Age (yr) | 41 | 48 | 57 | 54 | 67 |
| Gender | Male | Male | Female | Male | Male |
| Etiology | Alcohol | Alcohol | Hepatitis B | Alcohol | Hepatitis B |
| Child–Pugh class | C | C | B | B | B |
| Exudate | Macular and peripheral; bilateral | Macular;unilateral | Bilateral | Around macula; unilateral | Scattered;bilateral |
| Varices | Present1 | Present | Present | Present | Present |
| Glucose | 95 | 121 | 98 | 92 | 103 |
| Plt2 (/mm3) | 49 000 | 42 000 | 40 000 | 221 000 | 89 000 |
| PT (s) | 12.1 | 34.4 | 13.3 | 10.2 | 13.6 |
| Drugs3 | Present | Present | Present | Present | Present |
Retinopathy is not a well-studied feature of cirrhosis. Abe et al, compared retinal features of 85 hepatitis C patients with and without cirrhosis to those of control subjects (n = 100). They found that retinopathy comprises hemorrhage and exudates in 27 (31.8%) of patients. Risk factors were female gender, thrombocytopenia, systemic hypertension, a long history of liver disease, advanced age, and cirrhosis. Furthermore there was a statistically significant difference between the two groups (P<0.01)[4]. In our study we observed soft exudates only in the cirrhotic group. None of the healthy control subjects had exudates. This finding was statistically significant. We failed to find any statistically significant difference among Child class, platelet number, PT, and retinopathy. In addition to the results compatible with Abe’s study, we took a further step by excluding the two major causes of retinopathy, i.e., hypertension and DM. In Abe’s study eight patients with hepatitis C had evidence of cirrhosis. Five had hypertension and two had DM. Only two patients had neither DM nor hypertension. Therefore, our study is more focused on cirrhosis-related retinal changes by excluding hypertension and DM. In contrast to Abe’s study, none of our patients had hepatitis C infection; they had alcohol and/or hepatitis B instead. Abe et al, showed a waxing and waning pattern during IFN therapy and suggested a possible immune pathogenesis.
Pathophysiology of retinopathy is well defined in diabetics. Since retinopathy was not searched in cirrhotics in large surveys, pathophysiology involving retinopathy in cirrhosis is unknown. Increased estrogen formation may cause hormonal modification in retinopathy. Shunts in retinal vasculature resembling intrarenal and intrapulmonary shunts observed in hepatopulmonary syndrome and hepatorenal syndrome may be operational in retinal ischemia and cotton-wool spots. In our study none of the cirrhotics had hepatorenal syndrome. Our patients were not evaluated for the presence of diagnostic criteria for hepatopulmonary syndrome, but none of them had cyanosis and clubbing. Increased hydrostatic pressure caused by high portal pressure, hypoalbuminemia in cirrhosis and resultant decreased oncotic pressure may also contribute to exudate formation via extravasation of plasma contents. A study done by Dittmer and colleagues supports this suggestion[10]. Seventeen patients with cirrhosis and portal hypertension, largely due to alcohol consumption, had ophthalmic examination before and after transjugular intrahepatic portosystemic stent shunting. Retinopathy was evident in 11 patients of which 5 were exudate in nature. Retinopathy regressed significantly or disappeared completely after this procedure which has hemodynamic contributions to the systemic circulation. These findings were attributed to the fact that cirrhosis leads to decreased retinal perfusion.
We think retinal exudates and cotton-wool spots may represent pathologic manifestations of cirrhosis in patients without DM and hypertension. Retinopathy can be present not only in hepatitis C positive cases but also in other etiologies involved in cirrhosis. Although our findings are interesting further larger studies are warranted to reach a conclusion. Also, retinal examination of Child-Pugh class A group cirrhotics and those who do not have varices may reveal more information about impact of portal hypertension on retina. In conclusion, retinopathy leading to significant morbidity in DM may be observed even in cirrhotics without DM.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Fauci AS, Braunwald E, Isselbacher KJ. Harrison's principles of internal medicine. 14th ed. The McGraw Hill companies, 1998: 1710-1717. . |
| 2. | Ettl A, Daxecker F. Xerophthalmia in liver cirrhosis. Correct diagnosis after 15 years. Ophthalmologica. 1992;204:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | McClain CJ, Van Thiel DH, Parker S, Badzin LK, Gilbert H. Alterations in zinc, vitamin A, and retinol-binding protein in chronic alcoholics: a possible mechanism for night blindness and hypogonadism. Alcohol Clin Exp Res. 1979;3:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 92] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Abe T, Nakajima A, Satoh N, Koizumi T, Sakuragi S, Ono T, Komatsu M, Masamune O. Clinical characteristics of hepatitis C virus-associated retinopathy. Jpn J Ophthalmol. 1995;39:411-419. [PubMed] |
| 5. | Leonhartsberger J. Diabetic angiopathy and liver cirrhosis. Wien Med Wochenschr. 1978;128:17-19. [PubMed] |
| 6. | Pickup JC, Williams G. Textbook of Diabetes. 2nd ed. Blackwell Science Limited 1997: 44.1-45.1. . |
| 7. | Kanski JJ. Klinik oftalmoloji (Turkish translation). 4th ed. Butterworth-Heinemann, Nobel Tlp Kitabevleri (Turkish translation), 1999: 465-500. . |
| 8. | Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 Suppl 1:S5-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1635] [Cited by in RCA: 2107] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 9. | Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13416] [Cited by in RCA: 13270] [Article Influence: 603.2] [Reference Citation Analysis (0)] |
| 10. | Dittmer K, Nolte W, Tondrow M, Schwörer H. Pathologic fundus changes in advanced liver cirrhosis. Reduction of symptoms after portosystemic shunt. Ophthalmologe. 1998;95:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |