Published online Apr 14, 2005. doi: 10.3748/wjg.v11.i14.2174
Revised: September 8, 2004
Accepted: September 25, 2004
Published online: April 14, 2005
AIM: To analyze the risk factors of hepatocellular carcinoma (HCC) recurrence after radiofrequency ablation (RFA) treatment with HCV-associated hepatitis.
METHODS: Twenty-six patients with HCV-associated HCC who were followed-up for more than 12 mo were selected for this study. Risk factors for distant intrahepatic recurrences of HCC were evaluated for patients in whom complete coagulation was achieved without recurrence in the same subsegment as the primary nodule. Twelve clinical and tumoral factors were examined: Age, gender, nodule diameter, number of primary HCC nodule, Child-Pugh classification, serum platelet, serum albumin, serum AST, post RFA AST, serum ALT, post RFA ALT, post RFA treatment.
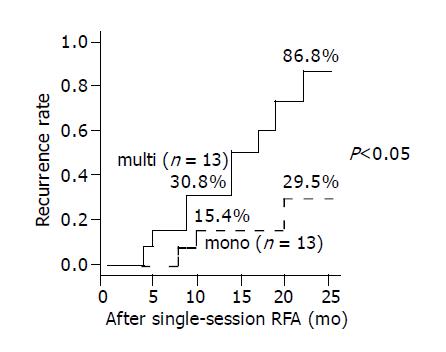
RESULTS: Distant recurrences of HCC in remnant liver after RFA were observed in 14 cases and in the number of primary HCC nodules (P = 0.047), and the serum platelets (P = 0.030), the clear difference came out by the recurrence group and the non-recurrence group. The cumulative recurrence rates after 1 and 2 years were 30.8% and 86.8%, respectively for primary multinodular HCC, and 15.4% and 29.5% respectively, for primary uninodular HCC. In addition the 1-year recurrence rates for patients with serum albumin more than 3.4 g/dL and less than 3.4 g/dL were 23.1% for both, but the 2-years recurrence rates were 89.0% and 23.1%, respectively. The number of primary HCC nodules (relative risk, 6.970; P = 0.016) were found to be a statistically significant predictor for poor distant intrahepatic recurrence by univariate analysis.
CONCLUSION: Patients who have multiple HCC nodules, low serum platelets and low serum albumin accompanied by HCV infection, should be carefully followed because of the high incidence of new HCC lesions in the remnant liver, even if coagulation RFA is complete.
- Citation: Yamanaka Y, Shiraki K, Miyashita K, Inoue T, Kawakita T, Yamaguchi Y, Saitou Y, Yamamoto N, Nakano T, Nakatsuka A, Yamakado K, Takeda K. Risk factors for the recurrence of hepatocellular carcinoma after radiofrequency ablation of hepatocellular carcinoma in patients with hepatitis C. World J Gastroenterol 2005; 11(14): 2174-2178
- URL: https://www.wjgnet.com/1007-9327/full/v11/i14/2174.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i14.2174
Various locoregional therapies including percutaneous ethanol injection (PEI), percutaneous acetic injection and radiofrequency ablation (RFA) were performed on hepatocellular carcinoma (HCC) patients with HCV-associated hepatitis. Radiofrequency ablation (RFA) is a novel means of treating patients with both metastatic and primary liver cancer[1]. It consists of a thermal treatment technique designed to produce approximately 3-cm diameter coagulative necrosis of the tissue in a single session. Moreover, performed RFA has been used for the treatment of HCC and can be percutaneously under local anesthesia[2]. A recent prospective study found that RFA requires fewer sessions such that PEI for patients with small HCCs and the 1-year survival rate is 98%[3]. Although these therapies can achieve complete necrosis of small HCC, its recurrence is still common. The intrahepatic recurrence rate is 20% during a mean follow-up period of 18 mo[4]. However, it is unclear which factors influence intrahepatic recurrence.
The purpose of this study was to analyze the risk factors of HCC recurrence after RFA treatment with HCV-associated hepatitis.
The clinical features of patients are summarized in Table 1. Among the patients diagnosed with HCC at the First Department of Internal Medicine, Mie University Hospital, from January 2001 to October 2003, 26 patients with HCV-associated HCC who were followed-up for more than 12 mo were selected for this study. Among them 12 patients had local recurrence in the other subsegment as the primary nodule, within as early as 6 mo evident from CT scans. All patients who were anti-HCV positive were evaluated. The 19 male and seven female patients had a median age of 67.9 years, and according to the Child-Pugh classification, 15 patients (58%) had class C cirrhosis and 11 patients (42%) had class B cirrhosis. There were 13 uninodular cases and 13 multinodular cases, and biopsy was not performed if the findings of ultrasonography (US), computed tomography (CT), and angiography were all indicative of HCC, and if AFP and DCP levels were elevated. The diagnosis of HCC was established by enhanced CT and CT during hepatic anteriography (CTA) and anterioportography (CTAP) and CTA and CTAP were performed on the patients before transcatheter arterial embolization (TAE) or (RFA). The median follow-up period was 12.5 mo with recurrence and 20 mo without recurrence.
| Gender | 1 |
| Number of males | 19(73%) |
| Number of females | 7(27%) |
| Median age | 67.9 |
| Child-Pugh A | 15(58%) |
| B | 11(42%) |
| C | 0(0%) |
| Number of HCC nodules | |
| Uninodular | 13(50%) |
| Multinodular | 13(50%) |
| Mean follow-up period (months) | |
| With recurrence | 12.5 |
| Without recurrence | 20 |
We utilized RFA for local ablation therapy of HCCs. Since the gelatin sponge remains in the tumor for 2 to 3 weeks after (TACE), RFA was generally performed within 2 weeks after TACE.
RFA was performed using a 17-gauge internally cooled straight electrode (Radionics, Burlington, MA). The patients received 35 mg of pethidine hydrochloride (Opystan, Tanabe, Tokyo, Japan) intravenously before RFA for analgesia, and antibiotics were administered before and 2 to 3 d after each chemoembolization or RFA procedure.
The RF electrode was inserted into the tumor under local anesthesia. The RF generator was then activated and the power needed to maintain a temperature of 90-120 °C at the tip was delivered for 10-20 min. The electrode was inserted into the tumor under real-time CT fluoroscopic guidance into patients for whom multiple electrode placements were required due to a large tumor size or because the entire lesion was not visualized by US imaging. Using CT fluoroscopy, bubble formation in the tumor does not prevent additional electrode insertion into the tumor and accumulation of iodized-oil in the tumor aids precise insertion of the electrode. The electrode was placed in the tumor depending on the tumor size and shape, and the RF generator was then activated at each tumor site. The endpoint of RFA was the presence of a well-defined area of no enhancing tissue including the treated tumor with a tumor-free margin of at least 5 mm in the arterial and portal phases of enhanced CT and as seen by the MR images.
Helical CT scan was used to determine the therapeutic effects. Complete coagulation was defined as no enhancement in the coagulated area on the helical CT scan one week after RFA. A follow-up CT scan was performed three months after RFA. As a rule thereafter, a helical CT scan was performed every 3 mo for at least 1 year. Local recurrence of an HCC nodule was defined as the development of an enhanced area on the CT scan in the same subsegment as the primary nodule. The helical CT scan was also used to study the distant recurrence of HCC in a different subsegment of the liver. Hepatic DSA was performed to confirm not only local but also distant recurrence of HCC.
Risk factors for the distant intrahepatic recurrence of HCC were evaluated for patients for whom complete coagulation was achieved without recurrence in the same subsegment as the primary nodule. Twelve clinical and tumor factors were examined: Age, gender, nodule diameter, number of primary HCC nodule, Child-Pugh classification, serum platelet, serum albumin, serum AST, post RFA serum AST, serum ALT, post RFA serum ALT.
The unpaired Student’s t-test was used to compare averages between groups and the χ2-test and Fisher’s exact probability test were used to compare independence. The distant intrahepatic recurrence rate was computed by Kaplan-Meier estimates and the Kaplan-Meier method and the log rank test were used to analyze the risk factors associated with the distant intrahepatic recurrences of HCC.
A stratified Cox proportional hazard regression model was used for multivariate analysis of age, gender, AST level as post RFA, platelet, Child-Pugh stage, nodule diameter, and the number of primary HCC. The results were reported as hazard ratios with 95% CI. A P-value of <0.05 was considered to be statistically significant for all analysis.
Among the 26 cases, the number of HCC uninodular and multinodular nodules were 13 and 13, respectively (Table 1), and distant recurrence of HCC in the remnant liver after RFA was observed for 14 cases. Clinical and tumoral charac-teristics were compared between the groups with and without distant intrahepatic recurrence. No statistically significant difference was observed with regard to age, gender, nodule diameter, Child-Pugh stage, serum albumin, serum pretr-eatment AST level, serum posttreatment AST level, serum pretreatment ALT levels, or the serum posttreatment AST level. In the number of primary HCC nodules, and the serum platelets, the difference came out intentionally by the recurrence group and the non-recurrence group.
In the number of primary HCC nodules (P = 0.047), and the serum platelets (P = 0.030), the clear difference came out by the recurrence group and the non-recurrence group. Also, no statistically significant difference was observed for UDCA therapy, and SNMC therapy after RFA treatment (Table 2). Since IFN therapy had few cases, it was not possible to compare it with other therapies about a curative effect.
| Variable | With recurrence(n = 12) | Without recurrence(n = 14) | P |
| Age (yr) | 67.8 ± 5.7 | 67.9 ± 7.8 | NS |
| Gender | |||
| Male | 6 | 11 | NS |
| Female | 6 | 3 | |
| Nodule diameter (mm) | 23.5 ± 7.2 | 32.0 ± 24.2 | NS |
| Number of primary | |||
| HCC nodules | |||
| Uninodular | 3 | 10 | <0.05 |
| Multinodular | 9 | 4 | |
| Child-Pugh A | 6 | 9 | NS |
| B | 6 | 5 | |
| Platelet (/mL) | 8.2 ± 3.6 | 13.3 ± 6.9 | <0.05 |
| Albumin (g/L) | 3.2 ± 0.3 | 3.4 ± 0.4 | NS |
| AST (IU/L) | 197.7 ± 416.0 | 87.2 ± 47.2 | NS |
| Post-RFA AST (IU/L) | 72.4 ± 25.4 | 70.8 ± 2.96 | NS |
| ALT (IU/L) | 98.0± 121.6 | 76 ± 43.5 | NS |
| Post-RFA ALT (IU/L) | 49.3 ±21.4 | 55.4 ± 30.5 | NS |
| Post-RFA treatment | |||
| UDCA | 10 | 12 | NS |
| SNMC | 5 | 4 | |
| IFN | 0 | 2 |
The longest duration of the case that we observed was 31 mo. Furthermore, the distant intrahepatic recurrence rates were analyzed based on the number of primary HCC nodules using the Kaplan-Meier method, and the recurrence rates after 1 and 2 years were 30.8%, and 86.8%, respectively for primary multinodular HCC, and 15.4% and 29.5%, respectively, for primary uninodular HCC. The primary multinodular HCC was significantly (P = 0.0136) associated with a higher distant intrahepatic recurrence rate compared with primary uninodular HCC (Figure 1). The 1-year recurrence rate for patients with serum albumin of more than 3.4 g/L and less than 3.4 g/L were 23.1% each for, but the 2-year recurrence rate they were 89.0% and 23.1%. Low (less than 3.4 g/L) serum albumin was significantly (P = 0.0236) associated with a higher recurrence rate compared with high (more than 3.4 g/L) serum albumin (Figure 2).
To search for more reliable prognostic markers, a stratified Cox proportional hazard regression model was created involving eight parameters associated with decreased distant intrahepatic recurrence. The number of primary HCC nodules (relative risk, 6.970; P = 0.016) was found to be statistically significant predictors for poor distant intrahepatic recurrence by multivariate analysis (Table 3).
| Variables CI | Ungav. /fav.1 | Relative risk | CI | P |
| Multinodular | Multinodular/ Uninodular | 6.970 | 1.443-33.667 | 0.016 |
| Post-RFA AST2 | >40/< = 40 (IU/L) | 2.438 | 0.213-27.956 | 0.474 |
| Platelet2 | 10>/> = 10 (/mL) | 2.426 | 0.445-13.222 | 0.306 |
| Nodule diameter2 | 30>/> = 30 (mm) | 1.032 | 0.217-4.903 | 0.969 |
| Child-Pugh | B/A | 0.861 | 0.184-4.025 | 0.849 |
| Gender | Males/female | 0.627 | 0.132-2.986 | 0.558 |
| Age (yr)2 | 68>/< = 68 (yr) | 0.406 | 0.076-2.169 | 0.292 |
In this study, the rates of local and distal recurrence were 0% and 53.8% for primary HCC after RFA treatment.
Some studies reported local recurrence rates that vary from 45% to 53% for HCC patiants after RFA[22,24,25]. RFA is successful for achieving a one-session treatment for patients with small HCC using RFA with CT assistance, and RFA with CT assistance is effective for the treatment of patients with small HCC[5-21]. One of the advantages of RFA is that it can be repeatedly performed, can be combined with TACE, and can also be used according to the features of the disease and the response[7]. For patients with cirrhosis and HCC, RFA results in effective local control of disease in a significant proportion of patients and can be safely performed with minimal complications[8]. By virtue of its local curability, minimal effect on liver function, and easy repeatability for recurrence, image-guided percutaneous tumor ablations, especially RFA, will become increasingly important for the treatment of HCC[21]. RFA is a useful primary therapy for the treatment of patients with HCC, especially for cases with a poor liver reserve from cirrhosis and with multiple and deep-sited lesions. RFA also is an effective and relatively simple procedure for the treatment of liver cancers[6].
Using a variety of approaches, others have also reported high local recurrences after RFA in patients with HCC[22-24]. In contrast, our data with a local recurrence rate of 0% were lower than that of other studies[22-24]. TACE and RFA combination therapy and real-time evaluation of RFA effective area by CT in our methods provably contribute to this low local recurrence rate. Although recurrences are frequent due to various factors, for instance tumor size and the follow-up period, this study found that the distal recurrence rate is 53% for primary HCC after RFA treatment, and this result is similar to that of other studies[22-24].
In the number of primary HCC nodules (P = 0.047), and the serum platelets (P = 0.030), the difference came out intentionally by the recurrence group and the non-recurrence group. The number of primary HCC nodules (relative risk, 6.970; P = 0.016) was also found to be statistically significant predictors for poor distant intrahepatic recurrence by multivariate analysis. We also discovered that the primary serum albumin in patients with HCV-associated hepatitis after RFA treatment is associated with the development of distal recurrence rate.
The most important variable, which influences the local recurrence rate, is tumor size[14]. Patients who have more than two HCC nodules accompanied by HCV infection should be carefully followed because of a high incidence of new HCC lesions in the remnant liver, even if coagulation by microwave or ablation by radiofrequency is complete[11].
Our observations are consistent with these reports, and it is reasonable to argue that there may be multicentric occurrence of HCC in the remnant liver at the same frequency as that for HCV-associated cirrhosis.
In the current study, no statistically significant difference was observed in the serum pre-treatment and post-treatment AST level. The serum AFP level (relative risk, 0.621; P = 0.596; not shown) was not found to be a statistically significant predictor of poor distant intrahepatic recurrence by multivariate analysis.
The high serum ALT level for HCV-associated cirrohsis is associated with the rapid development of HCC[18]. Continuous elevation of ALT is important for HCC diagnosis. Patients with the high level of serum ALT for 2 years or more were at a greater risk of HCC development[17]. Patients with HCV-associated cirrhosis with a sustained high serum ALT level are at a high risk of HCC development, suggesting the possibility of the prevention of HCC development in HCV-associated cirrhosis patients by reducing inflammatory necrosis[13]. Many investigators have shown that patients with cirrhosis who have persistently high ALT levels have a high risk of developing HCC[16,19,20]. It was necessary to continue treatment with anti-inflammatory drugs following initial IFN therapy to suppress ALT below 80 IU. This prevents HCC occurrence or delays the time of HCC occurrence and prolongs patient’s life span[17].
In the present study, no statistically significant difference was observed between the serum pretreatment and post-treatment AST levels and serum pretreatment and post-treatment ALT levels, indicating a high incidence of HCC recurrence in HCV-associated cirrhotic patients.
Recently, the risk of HCC occurrence was discovered to increase with the liver fibrosis stage in HCV-related liver disease[9,10]. The intrahepatic recurrence rate in C-viral and B-viral HCC is higher than that in NBNC-related HCC. Furthermore, fibrosis staging and the pathological grading of HCC are significantly linked to intrahepatic recurrence as seen by univariate analysis[13].
We did not evaluate the liver fibrosis stage for HCV-related liver disease. The serum platelet count (P = 0.030) for the group with recurrence was significantly higher than that for the group without recurrence. Compared with a group with the serum high platelet value, the group with a low platelet value of the recurrence rate of the HCC is clearly higher. The low level (less than 3.4 g/L) of serum albumin was significantly (P = 0.0236) associated with a higher recurrence rate compared with the high level (more than 3.4 g/L) of serum albumin, and this result is similar to those of previous studies[16,17,19,20]. These results suggest that the progression of fibrosis increases the recurrence of HCC with HCV-associated cirrhosis.
Serum AFP levels are significantly linked to intrahepatic recurrence as seen by univariate analysis[13]. Specifically, the level of lectin-reactive AFP is a suitable predictive marker for the early recognition of HCC during the follow-up of patients with cirrhosis[12]. In this study, no statistically significant difference was observed for the serum AFP level.
In conclusion, patients who have multiple HCC nodules, low serum platelets and serum albumin less than 3.4 g/L accompanied by HCV infection should be carefully followed, because of a high incidence of new HCC lesions in the remnant liver even if coagulation RFA is complete.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Fontana RJ, Hamidullah H, Nghiem H, Greenson JK, Hussain H, Marrero J, Rudich S, McClure LA, Arenas J. Percutaneous radiofrequency thermal ablation of hepatocellular carcinoma: a safe and effective bridge to liver transplantation. Liver Transpl. 2002;8:1165-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Francica G, Marone G. Ultrasound-guided percutaneous treatment of hepatocellular carcinoma by radiofrequency hyperthermia with a 'cooled-tip needle'. A preliminary clinical experience. Eur J Ultrasound. 1999;9:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Yamakado K, Nakatsuka A, Ohmori S, Shiraki K, Nakano T, Ikoma J, Adachi Y, Takeda K. Radiofrequency ablation combined with chemoembolization in hepatocellular carcinoma: treatment response based on tumor size and morphology. J Vasc Interv Radiol. 2002;13:1225-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Yamakado K, Nakatsuka A, Akeboshi M, Shiraki K, Nakano T, Takeda K. Combination therapy with radiofrequency ablation and transcatheter chemoembolization for the treatment of hepatocellular carcinoma: Short-term recurrences and survival. Oncol Rep. 2004;11:105-109. [PubMed] |
| 5. | Yamasaki T, Kurokawa F, Shirahashi H, Kusano N, Hironaka K, Okita K. Percutaneous radiofrequency ablation therapy with combined angiography and computed tomography assistance for patients with hepatocellular carcinoma. Cancer. 2001;91:1342-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Jiang HC, Liu LX, Piao DX, Xu J, Zheng M, Zhu AL, Qi SY, Zhang WH, Wu LF. Clinical short-term results of radiofrequency ablation in liver cancers. World J Gastroenterol. 2002;8:624-630. [PubMed] |
| 7. | Livraghi T. Radiofrequency ablation, PEIT, and TACE for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2003;10:67-76. [PubMed] |
| 8. | Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 530] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 9. | Takano S, Yokosuka O, Imazeki F, Tagawa M, Omata M. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatology. 1995;21:650-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 243] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, Inoue O, Yano M, Tanaka M, Fujiyama S. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. IHIT Study Group. Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann Intern Med. 1999;131:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 778] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 11. | Izumi N, Asahina Y, Noguchi O, Uchihara M, Kanazawa N, Itakura J, Himeno Y, Miyake S, Sakai T, Enomoto N. Risk factors for distant recurrence of hepatocellular carcinoma in the liver after complete coagulation by microwave or radiofrequency ablation. Cancer. 2001;91:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Shiraki K, Takase K, Tameda Y, Hamada M, Kosaka Y, Nakano T. A clinical study of lectin-reactive alpha-fetoprotein as an early indicator of hepatocellular carcinoma in the follow-up of cirrhotic patients. Hepatology. 1995;22:802-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 127] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Koike Y, Shiratori Y, Sato S, Obi S, Teratani T, Imamura M, Hamamura K, Imai Y, Yoshida H, Shiina S. Risk factors for recurring hepatocellular carcinoma differ according to infected hepatitis virus-an analysis of 236 consecutive patients with a single lesion. Hepatology. 2000;32:1216-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Hori T, Nagata K, Hasuike S, Onaga M, Motoda M, Moriuchi A, Iwakiri H, Uto H, Kato J, Ido A. Risk factors for the local recurrence of hepatocellular carcinoma after a single session of percutaneous radiofrequency ablation. J Gastroenterol. 2003;38:977-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 184] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Pompili M, Rapaccini GL, de Luca F, Caturelli E, Astone A, Siena DA, Villani MR, Grattagliano A, Cedrone A, Gasbarrini G. Risk factors for intrahepatic recurrence of hepatocellular carcinoma in cirrhotic patients treated by percutaneous ethanol injection. Cancer. 1997;79:1501-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Ikeda K, Saitoh S, Arase Y, Chayama K, Suzuki Y, Kobayashi M, Tsubota A, Nakamura I, Murashima N, Kumada H. Effect of interferon therapy on hepatocellular carcinogenesis in patients with chronic hepatitis type C: A long-term observation study of 1,643 patients using statistical bias correction with proportional hazard analysis. Hepatology. 1999;29:1124-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 362] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 17. | Mahmood S, Niiyama G, Kawanaka M, Nakata K, Sho M, Yasuhara Y, Ito T, Yamada G. Long term follow-up of a group of chronic hepatitis C patients treated with anti-inflammatory drugs following initial interferon therapy. Hepatol Res. 2002;24:213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Tarao K, Rino Y, Ohkawa S, Shimizu A, Tamai S, Miyakawa K, Aoki H, Imada T, Shindo K, Okamoto N. Association between high serum alanine aminotransferase levels and more rapid development and higher rate of incidence of hepatocellular carcinoma in patients with hepatitis C virus-associated cirrhosis. Cancer. 1999;86:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Lin SM, Lin CJ, Hsu CW, Tai DI, Sheen IS, Lin DY, Liaw YF. Prospective randomized controlled study of interferon-alpha in preventing hepatocellular carcinoma recurrence after medical ablation therapy for primary tumors. Cancer. 2004;100:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Shiratori Y, Shiina S, Teratani T, Imamura M, Obi S, Sato S, Koike Y, Yoshida H, Omata M. Interferon therapy after tumor ablation improves prognosis in patients with hepatocellular carcinoma associated with hepatitis C virus. Ann Intern Med. 2003;138:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 217] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 21. | Shiina S, Teratani T, Obi S, Hamamura K, Koike Y, Omata M. Nonsurgical treatment of hepatocellular carcinoma: from percutaneous ethanol injection therapy and percutaneous microwave coagulation therapy to radiofrequency ablation. Oncology. 2002;62 Suppl 1:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381-391. |
| 23. | Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, Pignata S, Daniele B, Cremona F. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 805] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 24. | Tranberg KG. Percutaneous ablation of liver tumours. Best Pract Res Clin Gastroenterol. 2004;18:125-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Rossi S, Garbagnati F, Lencioni R, Allgaier HP, Marchianò A, Fornari F, Quaretti P, Tolla GD, Ambrosi C, Mazzaferro V. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 345] [Article Influence: 13.8] [Reference Citation Analysis (0)] |