Published online Apr 14, 2005. doi: 10.3748/wjg.v11.i14.2080
Revised: November 10, 2004
Accepted: November 23, 2004
Published online: April 14, 2005
AIM: Clinical application of human hepatocytes (HC) is hampered by the progressive loss of growth and differentiation in vitro. The object of the study was to evaluate the effect of a biphasic culture technique on expression and activation of growth factor receptors and differentiation of human adult HC.
METHODS: Isolated HC were sequentially cultured in a hormone enriched differentiation medium (DM) containing nicotinamide, insulin, transferrin, selenium, and dexame-thasone or activation medium (AM) containing hepatocyte growth factor (HGF), epidermal growth factor (EGF), and granulocyte-macrophage colony-stimulating factor (GM-CSF). Expression, distribution and activation of the HC receptors (MET and EGFR) and the pattern of characteristic cytokeratin (CK) filaments were measured by fluorometry, confocal microscopy and Western blotting.
RESULTS: In the biphasic culture system, HC underwent repeated cycles of activation (characterized by expression and activation of growth factor receptors) and re-differentiation (illustrated by distribution of typical filaments CK-18 but low or absent expression of CK-19). In AM increased expression of MET and EGFR was associated with receptor translocation into the cytoplasm and induction of atypical CK-19. In DM low expression of MET and EGFR was localized on the cell membrane and CK-19 was reduced. Receptor phosphorylation required embedding of HC in collagen type I gel.
CONCLUSION: Control and reversible modulation of growth factor receptor activation of mature human HC can be accomplished in vitro, when defined signals from the extracellular matrix and sequential growth stimuli are provided. The biphasic technique helps overcome de-differentiation, which occurs during continuous stimulation by means of growth factors.
- Citation: Auth MK, Boost KA, Leckel K, Beecken WD, Engl T, Jonas D, Oppermann E, Hilgard P, Markus BH, Bechstein WO, Blaheta RA. Controlled and reversible induction of differentiation and activation of adult human hepatocytes by a biphasic culture technique. World J Gastroenterol 2005; 11(14): 2080-2087
- URL: https://www.wjgnet.com/1007-9327/full/v11/i14/2080.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i14.2080
Liver regeneration requires the remaining hepatocytes (HC) not only to retain their ability to proliferate but also to perform all essential functions needed for homeostasis in vivo[1]. In recent years, some obstacles encountered during the culturing of human liver cells have been overcome, emphasizing the role of soluble growth factors[2], extracellular matrix (ECM)[3,4], cellular cross-talk[5-7] and the existence of a compartment of progenitor cells[6]. However, little attention has been attributed to the dynamics of this process, which requires adaptation of parenchymal cells to systemic signals and changes in their microenvironment. Sequential coordination of this complex process finally determines if successful regeneration (growth and repair) or irreversible liver failure occurs.
Current in vitro models have suggested that in vitro growth of HC involves de-differentiation, characterized by induction of alpha-fetoprotein (AFP) or cytokeratin 19 (CK-19)[2,3,8,9]. In human liver, CK-19 is expressed in fetal hepatic progenitor cells and in mature bile duct epithelial cells, but not in mature HC[10]. Models of HC transplantation suggest that HC proliferation in the adult liver is not associated with de-differentiation[9]. In cell culture models with rat HC, proliferation triggered by hepatocyte growth factor (HGF) and epidermal growth factor (EGF) has been associated with the loss of liver-specific proteins [albumin, cytochrome P450 (CY P450)] and induction of AFP and CK-19[2,3,5,11,12]. Re-differentiation to the HC phenotype has been attributed to the presence of either EHS gel, a basement membrane analogue from the Engelbreth-Holm-Swarm mouse sarcoma, which contains several ECM components and growth factors[2], or collagen type I gel[8]. Growth arrest has been associated with re-differentiation, possibly as a result of decreased expression of growth receptors for EGF (EGFR) and HGF (MET)[2,4].
However, in these systems, the continuous exposure to hepatic mitogens is not physiologic and does not reflect the situation in vivo, where paracrine secretion of growth factors and matrix deposition occur in a sequential manner[13]. With regard to organ development, it is well established that tyrosine kinase receptors are critical components in the pertinent response to mesenchymal signals, requiring receptor expression at the appropriate times[14]. In current culture models, continuous exposure of cultured HC to high levels of HGF and EGF has enabled substantial proliferation of rodent HC in vitro[2], but subsequent transmission of this model to human HC in vitro has not yielded comparable results[4]. Decrease in tyrosine phosphorylation and down-regulation of receptor proteins have been suggested to account for the decline, but as yet this dilemma has not been resolved.
Expansion of mature human HC in vitro and controlling their differentiation is essential to employing human liver cells in bioartificial liver support, would help improve the outcome of gene therapy and HC transplantation and would open new perspectives for stem cell engineering. In this paper we describe a novel, defined culture model based upon a biphasic culture technique of human HC. We have analyzed the effects of culture medium providing either factors of metabolic support (differentiation medium (DM), containing dexamethasone, insulin, transferrin, selenium, and high amounts of nicotinamide) or mitogenic stimulation (activation medium (AM), containing moderate amounts of EGF, HGF, granulocyte-macrophage colony-stimulating factor (GM-CSF)) onto expression and bioactivity of MET and EGFR in human HC. We have examined receptor expression, distribution (membrane or cytoplasmic) and functional activity (phosphorylation) of these receptors and the corresponding phenotype of these cells (pattern of CK-18 and CK-19).
We provide compelling evidence that control of differentiation and activation of human HC is feasible in a reversible manner by sequential modulation of soluble nutrients. In contrast to other models, we demonstrate that biological response of human HC to soluble growth factors is not inhibited, but supported by a surrounding 3D ECM, if chemically defined collagen type I gel is applied.
For isolation of primary human HC, normal human liver tissue was obtained from hemihepatectomy specimen (due to secondary liver metastases) or from unused liver transplant tissue (reduced sized grafts). For cell isolation purposes, the normal hepatic tissue was carefully resected at a safe distance to the tumor tissue and the resection edge. The patients’ sex was equally distributed, their age ranged from 20 to 60 years, and tissue was only isolated if primary liver disease was excluded. Informed consent was obtained, and the study was approved by the local ethical committee in accordance with the Declaration of Helsinki. Human HC were isolated by the two-step collagenase perfusion technique and seeded on a 2D collagen I matrix or within a 3D collagen sandwich as described elsewhere[8].
HC were cultured continuously either in a chemically defined DM or AM. Both media consisted of DMEM (Invitrogen, Karlsruhe, Germany), supplemented with 50 mL/L pooled human serum, 20 mmol/L Hepes, and 50 µg/mL gentamycin. DM was enriched with nicotinamide, insulin, transferrin, selenium, and dexamethasone. AM was deprived of hormones but instead enriched with the mitogens EGF, HGF, and GM-CSF. The full description of both media is shown in Table 1.
| DM | AM | |
| DMEM | DMEM/Ham’s F12 | |
| 20 mmol/L Hepes | 20 mmol/L Hepes | |
| 50 µg/mL gentamycin | 50 µg/mL gentamycin | |
| 50 mL/L human serum | 50 mL/L human serum | |
| 10 ng/mL EGF | ||
| 1 ng/mL HGF | ||
| 1 ng/mL GM-CSF | ||
| D-glucose | 2 g/L | 3.15 g/L |
| Galactose | 2 g/L | |
| Ornithine | 0.1 g/L | |
| L-proline | 0.03 g/L | 0.02 g/L |
| Nicotinamide | 305 mg/L | 2.02 mg/L |
| ZnCl2 | 0.544 mg/L | |
| ZnSO4·7H2O | 0.75 mg/L | 0.432 mg/L |
| CuSO4·5H2O | 0.2 mg/L | 0.0013 mg/L |
| MnSO4 | 0.025 mg/L | |
| L-glutamine | 580 mg/L | 365 mg/L |
| ITS1 | 0.5 g/L | |
| Dexamethasone | 1.0 g/L |
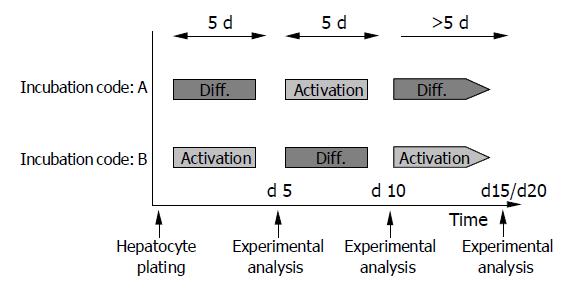
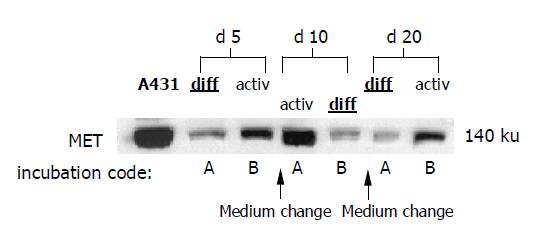
In subsequent studies, both media were used alternately in 5-d intervals. Freshly isolated HC were incubated with DM (incubation code A) or AM (incubation code B) for 5 d, followed by a switch to AM (incubation code A) or DM (incubation code B) for the next 5 d. Then a further medium change was carried out to recover the initial culture conditions. Figure 1 shows the experimental design.
Receptor surface expression was detected using a FACscan (Becton Dickinson, Heidelberg, Germany)[8]. The following antibodies were used: anti-MET (rabbit, polyclonal; Santa Cruz Biotechnology, Heidelberg, Germany), anti-EGFR (mouse, clone LA22; Santa Cruz Biotechnology), anti-cytokeratin 18 (mouse, clone Ks18.04; Progen, Heidelberg, Germany), anti-cytokeratin 19 (mouse, clone Ks19.1; Progen). FITC-conjugated goat-anti-rabbit IgG or goat anti-mouse IgG served as the secondary antibody.
HC were plated on round cover slips (pretreated with collagen I) and incubated for 60 min with unconjugated anti-MET or anti-EGFR antibody. Indocarbocyanine (Cy 3TM; Dianova; working dilution: 1:50) conjugated goat-anti-mouse IgG or goat anti-rabbit IgG was added as the secondary antibody. Specimens were viewed after embedding in an antifade reagent/mounting medium mixture (ProLongTM Antifade Kit, MoBiTec, Göttingen, Germany) using a confocal laser scanning microscope (LSM 10; Zeiss, Jena, Germany)[6].
HC lysates were applied to a 7% polyacrylamide gel and electrophoresed for 90 min at 60 V. The protein was then transferred to nitrocellulose membranes. After blocking, the membranes were incubated overnight with the anti-MET (dilution 1:10000) or anti-EGFR (dilution 1:100) antibody. HRP-conjugated goat-anti-mouse IgG (Upstate Biotechnology, Lake Placid, NY; dilution 1:5000) served as the secondary antibody. The membrane was briefly incubated with ECL detection reagent (ECLTM, Amersham) to visualize the proteins and exposed to an X-ray film (HyperfilmTM ECTM, Amersham). The cell line A431 served as the positive control.
At several time points after plating, cell culture medium was removed and replaced by a serum- and growth-factor-free medium. After 20-h incubation, HC were stimulated for 3 min with either 10 ng/mL EGF or 1 ng/mL HGF. Subsequently, HC were rinsed with ice-cold PBS, lysed for 5 min in lysis buffer and the Western blot assay was carried out as described above. The monoclonal antibody p-Tyr (Santa Cruz Biotechnology; clone PY99, 1:250 dilution) was used to detect ligand-induced tyrosine phosphorylation.
All experiments with flow cytometry were performed six times. Data presented in the graphics are expressed as mean±SD. Statistical analysis was conducted by the Wilcoxon-Mann-Whitney-U test. Differences were considered statistically significant at P<0.05.
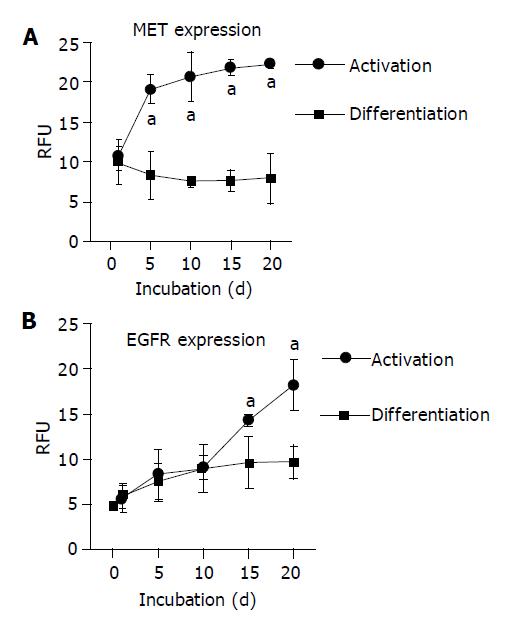
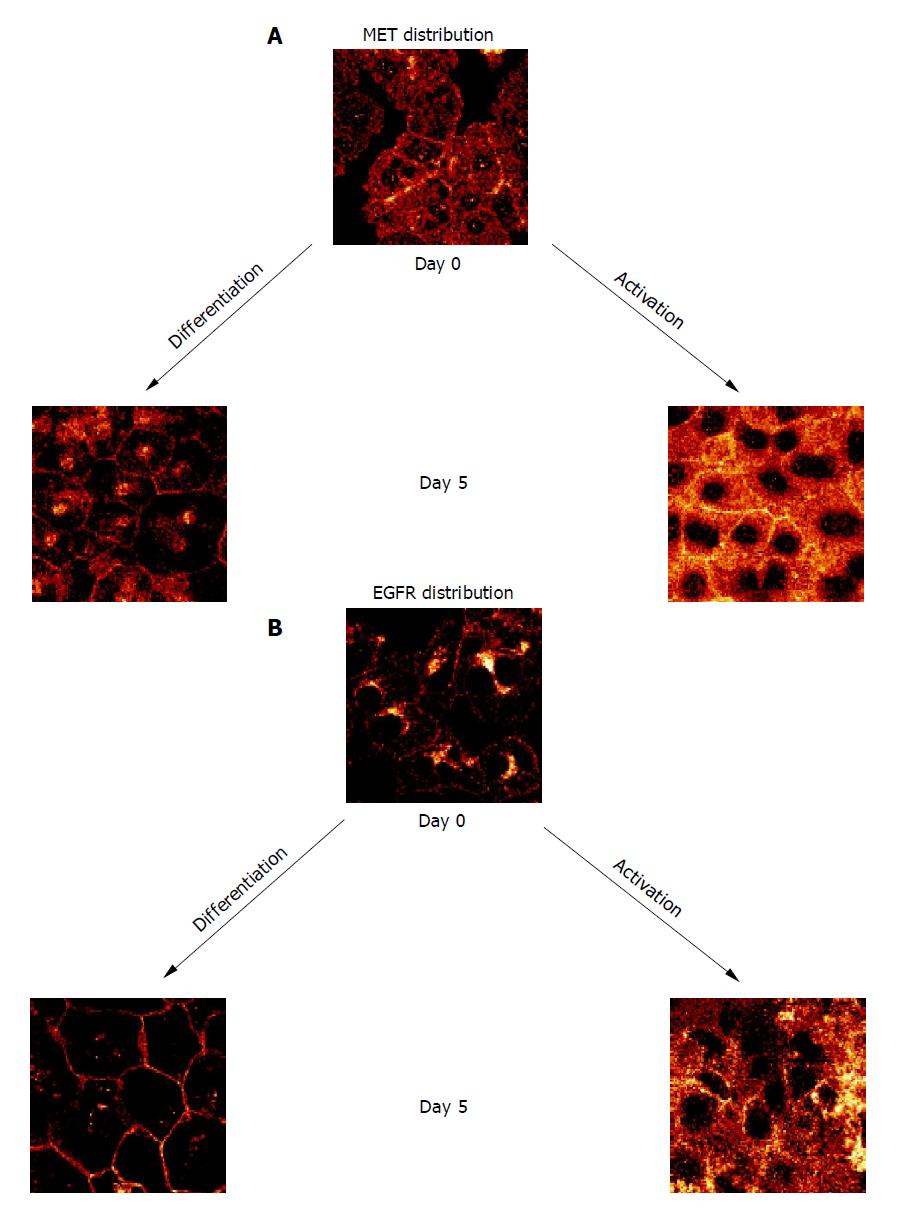
When HC were permanently incubated in AM, membranous MET expression rapidly increased and reached a plateau phase within 10 d. In contrast, MET expression remained low in the continuous presence of growth-factor-free DM (Figure 2A). Confocal examination of receptor distribution, 8 h after HC isolation, demonstrated weak receptor expression mainly along the plasma membrane. Prolonged cultivation in DM typically resulted in distinct surface assembly of MET. This pattern remained stable for 3 wk. A different feature was observed in the presence of AM, as MET strongly accumulated in the cytoplasm, already 5 d after plating out the HC (Figure 3A).
Contrary to the dynamic alterations of MET, membranous EGFR expression was not enhanced by growth factors during the early period of cell cultivation. Rather, EGFR was not up-regulated until after a 10-d lag phase (Figure 2B). Simultaneous confocal analysis showed initial cytoplasmic EGFR location, followed by a strong intracellular accumulation when cultivated in AM, or by a distinct enrichment at the hepatocellular membrane when cultivated in DM (Figure 3B).
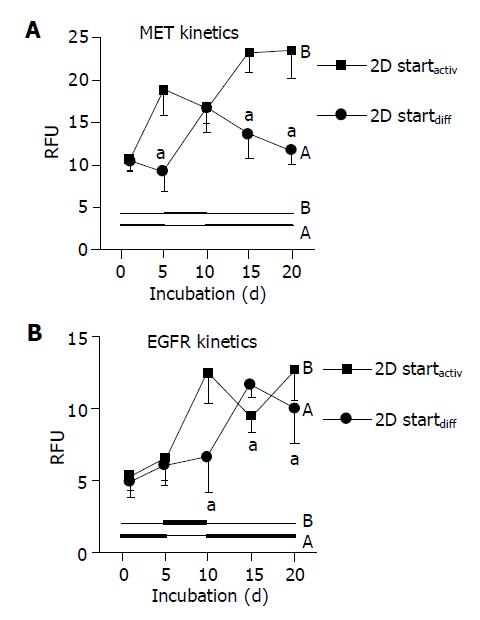
When using DM and AM alternately, MET and EGFR expression kinetics strictly followed the extracellular conditions. When HC were initially treated with DM for 5 d, MET remained constant during this time (Figure 4A, code A). However, the subsequent switch to AM evoked a nearly two-fold increase in MET by d 10. This process was reversible, as the MET level nearly returned to basal values when the initial culture conditions were established again (d 20). Reversal of the differentiation status was also possible when HC were cultured in the order AM-DM-AM (Figure 4A, code B).
In good accordance with the fluorometric analyses, Western blot data showed reversible changes of total MET protein content, which were strongly dependent on the cell culture conditions (Figure 5).
Similar changes were observed with respect to the EGFR. However, due to the lag phase of the EGFR expression kinetics, which has been demonstrated in Figure 2B, reversible receptor changes in response to the cell culture medium did not occur immediately (as seen with respect to MET expression), but with 5-10 d delay (Figure 4B).
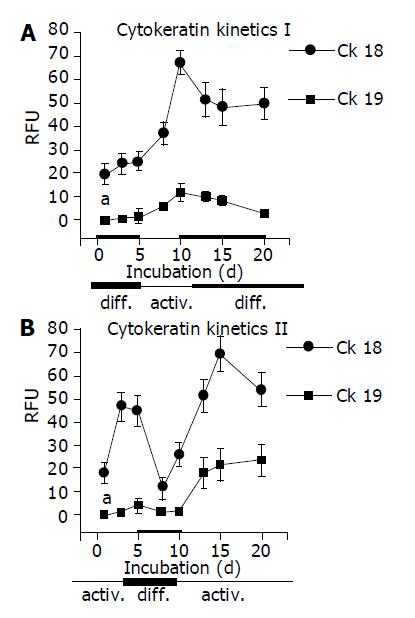
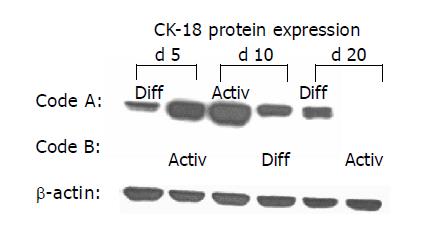
As CK are well-established differentiation markers, all experiments were repeated with respect to CK-18 and CK-19 expression. AM triggered up-regulation of CK-18 and de novo expression of CK-19. In contrast, DM evoked stable CK-18 expression level and prevented CK-19 synthesis. The processes were reversible, a switch from AM to DM was paralleled by a strong down-regulation of both CK-18 and CK-19. The subsequent switch to AM increased the filament expression level again. CK-18 and CK-19 were also regulated in a reversible manner when HC were cultured in the order DM-AM-DM (Figures 6 and 7).
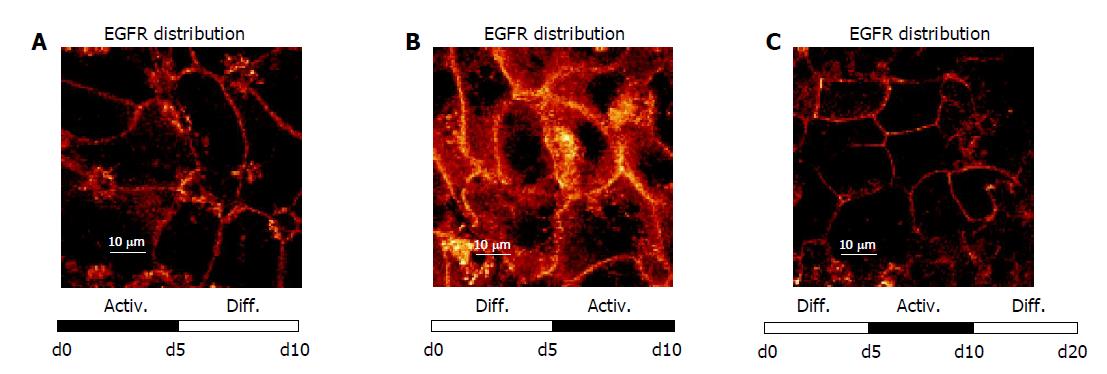
Figure 8 shows that EGFR localization followed the time-dependent medium changes in a reversible manner. When freshly isolated HC were initially incubated in AM up to d 5, and then exposed to DM (d 5 until d 10), intracellular EGFR became re-distributed along the plasma membrane (Figure 8A). In contrast, cultivation in the order DM up to d 5, AM from d 5 until d 10, was accompanied by a loss of EGFR surface expression, and a rapid internalization of this receptor (Figure 8B). A further switch back to DM was paralleled by a distinct re-accumulation of EGFR onto the surface of the plasma membrane (Figure 8C), which corresponds to the initial culture state.
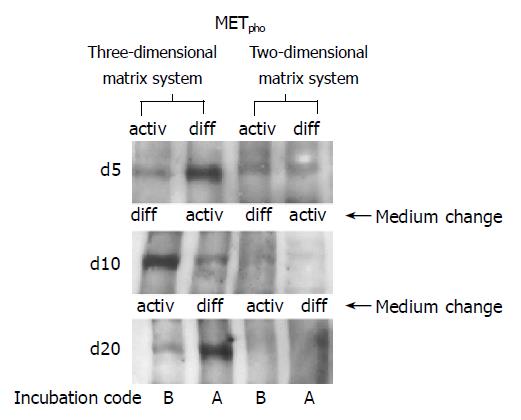
When HC were seeded on a 2D collagen matrix, only a weak MET phosphorylation signal was detected in the early cultivation period, independent of the cell culture medium used (Figure 9, right lanes, d 5). However, when HC retained their 3D structure by embedding them in a 3D collagen sandwich, a distinct activation of MET was evoked in response to a short-course stimulus by soluble HGF. The response was strongly dependent on the extracellular milieu. Figure 9, left lanes, demonstrates that MET phosphorylation was most prominent in a growth factor free, but hormone enriched environment DM. Receptor responsiveness could be induced in a controlled and reversible manner, as MET activity was high in presence of DM, was down-regulated in the presence of AM, but became reactivated when the starting conditions DM were re-introduced.
Over more than a decade there has been the hope of growing and expanding human HC in vitro[2,4,15,16]. While a medium with high amounts of HGF, EGF and nicotinamide has enabled proliferation of rodent HC[2], comparable results have not been achieved with human cells[4]. To prevent receptor down-regulation, which is associated with constant culture conditions, we developed a novel, biphasic culture technique. Hereby, we were able to demonstrate a controlled switch from hepatocellular differentiation to activation in a reversible manner.
The mixture of hormones and growth factors may exert counteracting effects in the downstream cascade, e.g., HGF and EGF activate the protein kinases ERK1/2, and the transcription factors NF-kappa B and AP-1, whereas dexamethasone inhibits them[17-19]. Therefore, we devised two distinct media (DM and AM) for alternate incubation.
Our composition of AM contained biologically active[20,21], but lower amounts of HGF, EGF, and nicotinamide than other models[2,4]. In order to synergize with HGF’s mode of action, we substituted with GM-CSF, which promotes activation of ERK1/2, NF-kappa B and AP-1[22-24]. In AM, we detected high receptor expression, but translocation into the cytoplasm, accompanied by increase of atypical intermediate filaments CK-19 (“ductular phenotype”), suggesting de-differentiation as described before[2,8].
Our composition of DM included dexamethasone, high levels of nicotinamide, insulin in a beneficial mixture with transferrin and selenium, metabolic substrates which have been shown to induce albumin expression, cytochrome P450 activity, and amino acid uptake[4,25-27]. We omitted HGF, which down-regulates CYP450-expression and synthesis of acute-phase proteins[11,12]. In DM, tyrosine kinase receptor expression was reduced, but accompanied by proper distribution along the plasma membrane. This correlated with reduction of atypical CK-19 expression, indicating more physiological differentiation (CK-18 positive, CK-19 negative) of parenchymal HC.
These observations suggest that continuous exposure to soluble mitogens does not go along with an adequate biological response. Conversely, presence of a growth-factor-free, but hormonally enriched environment DM supports not only cytoskeletal differentiation, but also enables appropriate receptor distribution.
To reflect variable metabolic demand and growth responsiveness, our dynamic model was based upon alternating stimulation by SM or DM in 5-d intervals. Hereby, we considered the dependency of liver-specific metabolic genes on hormonal changes[28], the capability of proliferation without shutting down liver-specific functions[1], the sequential course of organ development and regeneration in vivo[14], and the balanced interplay of positive and negative signals in this process[1].
It is possible that complete down-regulation of tyrosine kinase receptors was prevented in our system by lack of continuous exposure to mitogens and omission of nuclear hormones in AM[2,4]. Our activation interval was adjusted to the kinetics of liver cell proliferation in vivo[29] and in vitro[16,20,21]. In other models, continuously high mitogenic stimulation was associated with an increase of AFP and CK-19 over time[2,4], possibly reflecting a situation such as encountered in fulminant hepatitis, where very high levels of serum HGF induce ductular structures, eventually inhibiting liver regeneration and causing irreversible hepatic failure. Remarkably, in our model, re-differentiation could repeatedly be accomplished by a medium switch to DM. Therefore, our experiments indicate that an unphysiologic ductular phenotype may be induced by the continuous presence of growth factors. This process could be counteracted by a predominance of hormonal and metabolic substrates, suggesting that the maintenance of hepatic growth and metabolism may be feasible in vitro[9,28].
In our model, tyrosine kinase phosphorylation, physiologic expression of CK-18, and significant reduction of CK-19 levels depended on embedding in 3D collagen type I gel. It is well established that cell shape determines whether an individual cell will grow, differentiate, or die in response to growth factors[30]. Our findings are supported by previous reports, illustrating that collagen type I sandwich gel increased HC transcriptional activity and maintained liver-specific genes[31]. Furthermore, it has been tested as the only substrate enabling both maintenance of liver-specific genes and proliferation[1]. For the first time, we have been able to demonstrate the synergism of cell-matrix effects and soluble signals on differentiation and growth factor responsiveness of human HC.
The capacity for liver cell proliferation correlates with expression of MET, but not serum levels of HGF[32,33]. MET and EGFR are members of the transmembrane growth factor receptor tyrosine kinase family. Ligand-induced activation is characterized by receptor dimerization, autophosphorylation in the cytoplasmic domain, attracting cytosolic protein complexes, followed by phosphorylation of mitogen-activated protein kinase and kinases[14,28,34]. In our sequential model, a strong receptor expression associated with translocation into the cytoplasm was induced by the presence of exogenous growth factors. On the contrary, receptor expression was low, but distributed along the plasma membrane, when growth factors were omitted and a hormone-rich milieu was supplied. The appropriate localization and subsequent activation of growth receptors on human HC illustrate that essential biological requirements for liver repair and regeneration are provided by our culture model.
This biphasic technique represents progress in controlling growth and differentiation of human HC in vitro. Sequential stimulation of liver cells may help uncover the links between metabolism and the complex cascade of gene activation required for DNA replication[22]. Eventually, these studies might promote the application of human liver cells in HC transplantation, gene therapy, adult stem cell transfer, and bioartificial liver support[35,36].
We would like to thank Karen Nelson for critically reading the manuscript.
Science Editor Li WZ Language Editor Elsevier HK
| 1. | Bucher NL, Robinson GS, Farmer SR. Effects of extracellular matrix on hepatocyte growth and gene expression: implications for hepatic regeneration and the repair of liver injury. Semin Liver Dis. 1990;10:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Block GD, Locker J, Bowen WC, Petersen BE, Katyal S, Strom SC, Riley T, Howard TA, Michalopoulos GK. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J Cell Biol. 1996;132:1133-1149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 366] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 3. | Nishikawa Y, Tokusashi Y, Kadohama T, Nishimori H, Ogawa K. Hepatocytic cells form bile duct-like structures within a three-dimensional collagen gel matrix. Exp Cell Res. 1996;223:357-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Runge DM, Runge D, Dorko K, Pisarov LA, Leckel K, Kostrubsky VE, Thomas D, Strom SC, Michalopoulos GK. Epidermal growth factor- and hepatocyte growth factor-receptor activity in serum-free cultures of human hepatocytes. J Hepatol. 1999;30:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Michalopoulos GK, Bowen WC, Zajac VF, Beer-Stolz D, Watkins S, Kostrubsky V, Strom SC. Morphogenetic events in mixed cultures of rat hepatocytes and nonparenchymal cells maintained in biological matrices in the presence of hepatocyte growth factor and epidermal growth factor. Hepatology. 1999;29:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Mitaka T, Sato F, Mizuguchi T, Yokono T, Mochizuki Y. Reconstruction of hepatic organoid by rat small hepatocytes and hepatic nonparenchymal cells. Hepatology. 1999;29:111-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 178] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Michalopoulos GK, Bowen WC, Mulè K, Lopez-Talavera JC, Mars W. Hepatocytes undergo phenotypic transformation to biliary epithelium in organoid cultures. Hepatology. 2002;36:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Blaheta RA, Kronenberger B, Woitaschek D, Auth MK, Scholz M, Weber S, Schuldes H, Encke A, Markus BH. Dedifferentiation of human hepatocytes by extracellular matrix proteins in vitro: quantitative and qualitative investigation of cytokeratin 7, 8, 18, 19 and vimentin filaments. J Hepatol. 1998;28:677-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Dabeva MD, Laconi E, Oren R, Petkov PM, Hurston E, Shafritz DA. Liver regeneration and alpha-fetoprotein messenger RNA expression in the retrorsine model for hepatocyte transplantation. Cancer Res. 1998;58:5825-5834. [PubMed] |
| 10. | Van Eyken P, Sciot R, Callea F, Van der Steen K, Moerman P, Desmet VJ. The development of the intrahepatic bile ducts in man: a keratin-immunohistochemical study. Hepatology. 1988;8:1586-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 204] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Guillén MI, Gómez-Lechón MJ, Nakamura T, Castell JV. The hepatocyte growth factor regulates the synthesis of acute-phase proteins in human hepatocytes: divergent effect on interleukin-6-stimulated genes. Hepatology. 1996;23:1345-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Donato MT, Gómez-Lechón MJ, Jover R, Nakamura T, Castell JV. Human hepatocyte growth factor down-regulates the expression of cytochrome P450 isozymes in human hepatocytes in primary culture. J Pharmacol Exp Ther. 1998;284:760-767. [PubMed] |
| 13. | Yang Y, Spitzer E, Meyer D, Sachs M, Niemann C, Hartmann G, Weidner KM, Birchmeier C, Birchmeier W. Sequential requirement of hepatocyte growth factor and neuregulin in the morphogenesis and differentiation of the mammary gland. J Cell Biol. 1995;131:215-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 226] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Diehl AM, Rai RM. Liver regeneration 3: Regulation of signal transduction during liver regeneration. FASEB J. 1996;10:215-227. [PubMed] |
| 15. | Stolz DB, Michalopoulos GK. Comparative effects of hepatocyte growth factor and epidermal growth factor on motility, morphology, mitogenesis, and signal transduction of primary rat hepatocytes. J Cell Biochem. 1994;55:445-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Gómez-Lechón MJ, Guillén I, Ponsoda X, Fabra R, Trullenque R, Nakamura T, Castell JV. Cell cycle progression proteins (cyclins), oncogene expression, and signal transduction during the proliferative response of human hepatocytes to hepatocyte growth factor. Hepatology. 1996;23:1012-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Liu F, Su Y, Li B, Ni B. Regulation of amyloid precursor protein expression and secretion via activation of ERK1/2 by hepatocyte growth factor in HEK293 cells transfected with APP751. Exp Cell Res. 2003;287:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Thoresen GH, Guren TK, Christoffersen T. Role of ERK, p38 and PI3-kinase in EGF receptor-mediated mitogenic signalling in cultured rat hepatocytes: requirement for sustained ERK activation. Cell Physiol Biochem. 2003;13:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Kassel O, Sancono A, Krätzschmar J, Kreft B, Stassen M, Cato AC. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J. 2001;20:7108-7116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 368] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 20. | Ismail T, Howl J, Wheatley M, McMaster P, Neuberger JM, Strain AJ. Growth of normal human hepatocytes in primary culture: effect of hormones and growth factors on DNA synthesis. Hepatology. 1991;14:1076-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Strain AJ, Ismail T, Tsubouchi H, Arakaki N, Hishida T, Kitamura N, Daikuhara Y, McMaster P. Native and recombinant human hepatocyte growth factors are highly potent promoters of DNA synthesis in both human and rat hepatocytes. J Clin Invest. 1991;87:1853-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 140] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Fausto N. Liver regeneration. J Hepatol. 2000;32:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 911] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 23. | Bozinovski S, Jones JE, Vlahos R, Hamilton JA, Anderson GP. Granulocyte/macrophage-colony-stimulating factor (GM-CSF) regulates lung innate immunity to lipopolysaccharide through Akt/Erk activation of NFkappa B and AP-1 in vivo. J Biol Chem. 2002;277:42808-42814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Jubinsky PT, Short MK, Mutema G, Witte DP. Developmental expression of Magmas in murine tissues and its co-expression with the GM-CSF receptor. J Histochem Cytochem. 2003;51:585-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Inui T, Shinomiya N, Fukasawa M, Kobayashi M, Kuranaga N, Ohkura S, Seki S. Growth-related signaling regulates activation of telomerase in regenerating hepatocytes. Exp Cell Res. 2002;273:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Runge D, Runge DM, Jäger D, Lubecki KA, Beer Stolz D, Karathanasis S, Kietzmann T, Strom SC, Jungermann K, Fleig WE. Serum-free, long-term cultures of human hepatocytes: maintenance of cell morphology, transcription factors, and liver-specific functions. Biochem Biophys Res Commun. 2000;269:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Pacherník J, Esner M, Bryja V, Dvorák P, Hampl A. Neural differentiation of mouse embryonic stem cells grown in monolayer. Reprod Nutr Dev. 2002;42:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Taub R. Liver regeneration 4: transcriptional control of liver regeneration. FASEB J. 1996;10:413-427. [PubMed] |
| 29. | Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2649] [Cited by in RCA: 2467] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 30. | Mooney D, Hansen L, Vacanti J, Langer R, Farmer S, Ingber D. Switching from differentiation to growth in hepatocytes: control by extracellular matrix. J Cell Physiol. 1992;151:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 322] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 31. | Dunn JC, Tompkins RG, Yarmush ML. Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J Cell Biol. 1992;116:1043-1053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 227] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 32. | Tsubouchi H, Niitani Y, Hirono S, Nakayama H, Gohda E, Arakaki N, Sakiyama O, Takahashi K, Kimoto M, Kawakami S. Levels of the human hepatocyte growth factor in serum of patients with various liver diseases determined by an enzyme-linked immunosorbent assay. Hepatology. 1991;13:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 172] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | D'Errico A, Fiorentino M, Ponzetto A, Daikuhara Y, Tsubouchi H, Brechot C, Scoazec JY, Grigioni WF. Liver hepatocyte growth factor does not always correlate with hepatocellular proliferation in human liver lesions: its specific receptor c-met does. Hepatology. 1996;24:60-64. [PubMed] |
| 34. | Runge DM, Runge D, Foth H, Strom SC, Michalopoulos GK. STAT 1alpha/1beta, STAT 3 and STAT 5: expression and association with c-MET and EGF-receptor in long-term cultures of human hepatocytes. Biochem Biophys Res Commun. 1999;265:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Strain AJ, Neuberger JM. A bioartificial liver--state of the art. Science. 2002;295:1005-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 36. | Mahler S, Desille M, Frémond B, Chesné C, Guillouzo A, Campion JP, Clément B. Hypothermic storage and cryopreservation of hepatocytes: the protective effect of alginate gel against cell damages. Cell Transplant. 2003;12:579-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |