Published online Apr 7, 2005. doi: 10.3748/wjg.v11.i13.1991
Revised: March 28, 2004
Accepted: April 13, 2004
Published online: April 7, 2005
AIM: To evaluate the effects of percutaneous radiation on leukocyte-endothelium interaction (LEI) in experimental hepatocellular carcinoma (HCC).
METHODS: Twelve ACI rats underwent HCC-inoculation, six of which on day 12 received low-dose external radiation and six did not. After 12 h intravital microscopy was performed.
RESULTS: LEI was significantly reduced in tumor tissue. However, irradiation of liver sinusoids and tumor tissue with 6 Gy led to a significant activation of leukocyte adhesion in the tumor with a marked increase of the proinflammatory cytokine TNF-α.
CONCLUSION: The findings indicate that the immunological tumor-endothelial barrier can be overcome by external irradiation.
- Citation: Maksan SM, Schmidt E, Ryschich E, Harms W, Schmidt J. Enhancement of leukocyte adhesion after percutaneous irradiation in rats with hepatocellular carcinoma. World J Gastroenterol 2005; 11(13): 1991-1994
- URL: https://www.wjgnet.com/1007-9327/full/v11/i13/1991.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i13.1991
The incidence of hepatocellular carcinoma (HCC) is increasing worldwide, and represents the third most common cause of cancer-related death[1]. Although complete surgical resection is the primary goal, long-term survival is often limited by local recurrence or distant metastases of the tumor. Because of this challenge, there is a need to develop and evaluate new treatment options in HCC. At present, most patients are diagnosed when palliation with controversial survival benefits is the sole option[2]. Innovative therapeutic strategies are currently tested in HCC, including new biological target-based drugs, cyclooxygenase inhibitors, and gene therapy are currently tested in HCC[3].
Physiological and pathological vascularization events are not equivalent among organ systems, and the unique behavior of each system needs to be taken into account when evaluating angiogenic therapies[4]. Leukocyte-endothelium interaction (LEI) and migration could represent a worthwhile target for novel immunological treatment strategies as it represents the first and fundamental step in the immune cascade leading to tumor cell recognition and possible rejection. Without leukocyte adhesion to malignant tumor endothelium, there will be no migration into the tumor. That malignant tumors protect themselves by inhibiting leukocyte adhesion to their endothelial surface is a common phenomenon in pancreatic and other cancers[5-8].
LEI consists of the rolling of leukocytes along the vascular wall, and firm adherence of leukocytes to endothelial cells under certain conditions. This phenomenon is processed by cell adhesion molecules. Although significant progress has been made in understanding the underlying mechanisms of LEI, a therapeutical approach by enhancing the development of effective leukocyte infiltration in tumors is not established.
Irradiation of healthy tissues is known to upregulate endothelial adhesion molecules[9-11]. We hypothesized that a percutaneous single dose radiation of 6 Gy could enhance LEI within the tumor and induce intratumoral migration of leukocytes.
All experiments were performed with permission of the government authorities and in accordance with the German legislation on laboratory animal experiments (Regierun-gspräsidium Karlsruhe, Germany). Male ACI rats weighing 230.0±39.2 g (VAF-Plus, Harlan-Sprague Dawley, Indianapolis, USA) were used in all experiments. Animals were anesthetized with ketamine/pentobarbital (Ketanest S, Parke-Davis, Berlin, Germany and Narcoren, Merial, Hallbergmoos, Germany). The left carotid artery and jugular vein were cannulated for blood pressure measurement and application of FITC-labeled albumin and rhodamine 6G.
On day zero, intrahepatic tumor implantation of Morris hepatoma A3294 was performed in 12 ACI rats[12]. The recipient rats were anesthetized and the left liver lobe via a small midline incision was prepared for tumor implantation. Five million Morris hepatoma tumor cells (5 µL) were injected in subcapsular position. On d 12, six animals underwent a percutaneous single dose radiation with 6 Gy under general anesthesia. After 12 h the animals underwent laparotomy and intravital fluorescence microscopy was performed to determine tumor vessel diameter, red blood cell velocity (RBV) and leukocyte adherence[13]. Values were compared to control animals without irradiation.
All animals were killed at the end of videomicroscopy and the whole liver was harvested for histopathological investigations. Serum specimens were taken at the end of experiments for enzyme analysis and TNF-α values.
Intravital videomicroscopy was used according to the epiillumination technique reported by Menger et al[14]. In general anesthesia the left liver lobe was exteriorized after relaparotomy. A Leitz fluorescence microscope (Leitz GmbH, Wetzlar, Germany) was used. In the presence of different excitation filters (wavelength 450-490 and 530-560 nm) visualization of FITC-labeled erythrocytes (Fluorescein isothiocyanate Isomer 1, Sigma, St. Louis, USA) and leukocytes with rhodamine 6G (0.02 mg/kg body-weight, Sigma) was possible. For contrast enhancement of plasma, FITC-labeled albumin was administered intravenously during experiments (50 mg/kg body-weight, Sigma). The microscopy was videotaped and off-line analysis was performed using a computer-assisted processing system[15].
The following parameters were assessed in ten randomly selected tumor areas and fields of healthy liver tissue. RBV was measured using the frame to frame method offline. Volumetric blood flow (Vb) was visualized after intravenuous injection of FITC-labeled erythrocytes and analyzed offline. Determinants were erythrocyte velocity and vessel diameter (D) using the following equation: Vb = 15×Ve×π×D2[16]. LEI described the flow behavior of white blood cells and differentiated between low-affinity leukocytes (roller) moving with less than 66% of RBV or adhering for less than 30 s to the endothelium and high-affinity leukocytes (sticker) adhering for more than 30 s to the endothelium surface.
Blood gas analysis and monitoring of heart rate and mean arterial blood pressure were performed via the cannulated left carotid artery at the beginning of experiments, 30 min after the onset of videomicroscopy and 2 h after microscopy (ABL 5, Radiometer GmbH, Willich, Germany).
On d 13, blood samples of radiated animals and controls were taken after videomicroscopy for TNF-α measurement using a standardized ELISA kit (Pharmingen, USA).
One part of the harvested liver was fixed in buffered formalin and prepared for staining with hematoxilin and eosin to confirm tumor presence.
The data were expressed as mean±SD and compared between groups by Wilcoxon-Mann-Whitney U-test. P<0.05 was considered statistically significant.
There were no significant differences between the study groups in mean arterial blood pressure and blood gas analysis during intravital microscopy.
Control hemodynamics and blood gases were maintained at physiological levels throughout the experiments.
Vessel diameter and basal RBV were comparable in hepatic tumor tissue and healthy liver tissue (Tables 1 and 2). There was a homogenous but not significant increase in volumetric blood flow in both groups (Table 3).
| RBV | Healthy liver | Liver cancer |
| Controls | 1.49±0.3 | 1.85±0.12 |
| Radiation | 1.88±0.1 | 1.93±0.14 |
| Vessel diameter | Healthy liver | Liver cancer |
| Controls | 34.5±3.8 | 36.0±3.71 |
| Radiation | 35.6±2.82 | 36.5±0.92 |
| Blood flow | Healthy liver | Liver cancer |
| Controls | 0.25±0.07 | 0.29±0.02 |
| Radiation | 0.31±0.03 | 0.35±0.05 |
The number of high-affinity leukocytes was comparable in tumor tissue and healthy liver tissue (P>0.05) (Figure 1). After percutaneous low-dose irradiation (6 Gy), high-affinity LEI was significantly enhanced in tumor tissue and sinusoids (P<0.05) (Figure 1).
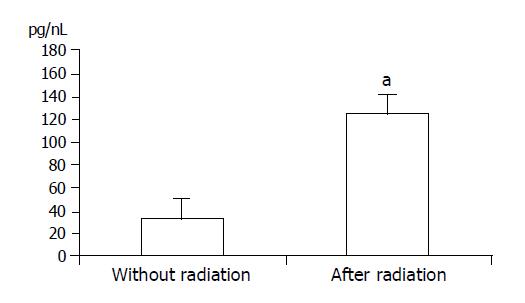
TNF-α levels were significantly elevated after radiation (P<0.05) (Figure 2).
The results of the current study indicate that LEI decreases significantly in tumor tissue under basal conditions, but this can be overcome by low-dose external radiation. We have previously shown that tumor-associated endothelial cells have a suppressed expression of ICAM-1 compared to endothelial cells from healthy liver[13]. Thus, only the basal expression of endothelial adhesion molecules is decreased in the tumor vasculature, but the possibility of inflammation-mediated upregulation is not hampered. The homogeneous LEI in liver sinusoids and tumor vessels after radiation indicates that endothelial cells can be activated probably by unspecific radiation-induced inflammation with a marked increase of proinflammatory cytokine TNF-α.
Radiotherapy with or without transarterial embolization and/or percutaneous ethanol injection appears effective in controlling HCC and can prolong survival[17] although this is still controversial. External beam radiation is rarely used as a single modality therapy as it has been shown that more than 50 Gy would be required to kill HCC cells. However, this radiation dose is commonly associated with radiation-induced hepatitis and liver failure when the whole liver is treated[18,19]. In contrast, high-dose conformal radiotherapy, including proton irradiation, has shown significant responses and acceptable toxicities by excluding the non-tumorous volume of the liver from the target volume. Local radiotherapy combined with transarterial catheter embolization (TACE) has also been investigated as a means of enhancing tumor control, because TACE has a limited effect on portal vein tumor thrombus and pericapsular invasion of the tumor. This approach may provide response rates of 50%[20,21] and overall survival benefit[22].
If tumor tissues are associated with tumor infiltrating lymphocytes at a high density or with sinus histiocytosis in its regional lymph nodes, good postoperative survival rates for cancer have been reported[23,24]. Involvement of an anti-tumor effect via cellular immunity, humoral immunity or via cytokines produced by the cancer cells has been discussed. TNF-α plays a critical role in the immune defense against tumor growth. By a regional infusion of the cytokine TNF-α and interferon-γ a significant reduction in tumor growth can be described in an animal model[25].
We focused on microcirculatory parameters and the course of TNF-α after radiation. Quantification of the LEI and determination of the proinflammatory cytokine TNF-α showed a significant increase after external single-dose radiation. These findings are in line with those of earlier studies, indicating that inflammation-mediated upregulation of adhesion molecules in tumor endothelium is possible[6,26].
Angiogenetic factors are capable of inducing a state of endothelial cell anergy. After activation by inflammatory cytokines there is a suppressed response of tumor endothelial cells compared to endothelial cells from normal tissue in human umbilical vein and human renal cell carcinoma[27]. This state is induced at the protein level (expression) and at the functional level (adhesion) and may serve as a tumor-protecting mechanism by impairing the development of efficient leukocyte infiltration into tumors[28,29].
In conclusion, percutaneous irradiation of the liver has an impact on the LEI in endothelium of HCC and healthy liver. This observation is important because it may allow to overcome an important immune escape mechanism of malignant endothelium, which is downregulated by adhesion molecules. These findings have to be further evaluated using additional immunologic effector cell stimulation in immunotherapeutic studies in HCC.
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2651] [Cited by in RCA: 2597] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 2. | Bruix J, Llovet JM. Prognostic assessment and evaluation of the benefits of treatment. J Clin Gastroenterol. 2002;35:S138-S142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Di Maio M, De Maio E, Perrone F, Pignata S, Daniele B. Hepatocellular carcinoma: systemic treatments. J Clin Gastroenterol. 2002;35:S109-S114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Ross MA, Sander CM, Kleeb TB, Watkins SC, Stolz DB. Spatiotemporal expression of angiogenesis growth factor receptors during the revascularization of regenerating rat liver. Hepatology. 2001;34:1135-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Piali L, Fichtel A, Terpe HJ, Imhof BA, Gisler RH. Endothelial vascular cell adhesion molecule 1 expression is suppressed by melanoma and carcinoma. J Exp Med. 1995;181:811-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 146] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Ryschich E, Harms W, Loeffler T, Eble M, Klar E, Schmidt J. Radiation-induced leukocyte adhesion to endothelium in normal pancreas and in pancreatic carcinoma of the rat. Int J Cancer. 2003;105:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Schmidt J, Ryschich E, Maksan SM, Werner J, Gebhard MM, Herfarth C, Klar E. Reduced basal and stimulated leukocyte adherence in tumor endothelium of experimental pancreatic cancer. Int J Pancreatol. 1999;26:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Shimoyama S, Gansauge F, Gansauge S, Widmaier U, Oohara T, Beger HG. Overexpression of intercellular adhesion molecule-1 (ICAM-1) in pancreatic adenocarcinoma in comparison with normal pancreas. Pancreas. 1997;14:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Hallahan D, Kuchibhotla J, Wyble C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res. 1996;56:5150-5155. [PubMed] |
| 10. | Quarmby S, Kumar P, Kumar S. Radiation-induced normal tissue injury: role of adhesion molecules in leukocyte-endothelial cell interactions. Int J Cancer. 1999;82:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Prabhakarpandian B, Goetz DJ, Swerlick RA, Chen X, Kiani MF. Expression and functional significance of adhesion molecules on cultured endothelial cells in response to ionizing radiation. Microcirculation. 2001;8:355-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Yang R, Rescorla FJ, Reilly CR, Faught PR, Sanghvi NT, Lumeng L, Franklin TD, Grosfeld JL. A reproducible rat liver cancer model for experimental therapy: introducing a technique of intrahepatic tumor implantation. J Surg Res. 1992;52:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Maksan SM, Paulo H, Ryschich E, Kuntz C, Gebhard MM, Klar E, Schmidt J. In vivo assessment of angioarchitecture and microcirculation in experimental liver cancer: a new model in rats. Dig Dis Sci. 2003;48:279-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Menger MD, Marzi I, Messmer K. In vivo fluorescence microscopy for quantitative analysis of the hepatic microcirculation in hamsters and rats. Eur Surg Res. 1991;23:158-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 78] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Zeintl H, Sack FU, Intaglietta M, Messmer K. Computer assisted leukocyte adhesion measurement in intravital microscopy. Int J Microcirc Clin Exp. 1989;8:293-302. [PubMed] |
| 16. | Endrich B. Hyperthermia and microcirculatory effects of heat in animal tumors. Recent Results Cancer Res. 1988;109:96-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Tokuuye K, Sumi M, Kagami Y, Murayama S, Kawashima M, Ikeda H, Ueno H, Okusaka T, Okada S. Radiotherapy for hepatocellular carcinoma. Strahlenther Onkol. 2000;176:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Lin DY, Lin SM, Liaw YF. Non-surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 1997;12:S319-S328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Cheng JC, Wu JK, Huang CM, Huang DY, Cheng SH, Lin YM, Jian JJ, Yang PS, Chuang VP, Huang AT. Radiation-induced liver disease after radiotherapy for hepatocellular carcinoma: clinical manifestation and dosimetric description. Radiother Oncol. 2002;63:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Ishikura S, Ogino T, Furuse J, Satake M, Baba S, Kawashima M, Nihei K, Ito Y, Maru Y, Ikeda H. Radiotherapy after transcatheter arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Clin Oncol. 2002;25:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Chia-Hsien Cheng J, Chuang VP, Cheng SH, Lin YM, Cheng TI, Yang PS, Jian JJ, You DL, Horng CF, Huang AT. Unresectable hepatocellular carcinoma treated with radiotherapy and/or chemoembolization. Int J Cancer. 2001;96:243-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Schwartz JD, Schwartz M, Mandeli J, Sung M. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: review of the randomised clinical trials. Lancet Oncol. 2002;3:593-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 182] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Svennevig JL, Lunde OC, Holter J, Bjørgsvik D. Lymphoid infiltration and prognosis in colorectal carcinoma. Br J Cancer. 1984;49:375-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 149] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 301] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 25. | Yang R, Liu Q, Rescorla FJ, Grosfeld JL. Experimental liver cancer: improved response after hepatic artery ligation and infusion of tumor necrosis factor-alpha and interferon-gamma. Surgery. 1995;118:768-772; discussion 772-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Ryschich E, Schmidt J, Loeffler T, Eble M, Gebhard MM, Harms W, Klar E. Different radiogenic effects on microcirculation in healthy pancreas and in pancreatic carcinoma of the rat. Ann Surg. 2003;237:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Griffioen AW, Damen CA, Blijham GH, Groenewegen G. Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood. 1996;88:667-673. [PubMed] |
| 28. | Griffioen AW, Damen CA, Martinotti S, Blijham GH, Groenewegen G. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res. 1996;56:1111-1117. [PubMed] |
| 29. | Wu NZ, Klitzman B, Dodge R, Dewhirst MW. Diminished leukocyte-endothelium interaction in tumor microvessels. Cancer Res. 1992;52:4265-4268. [PubMed] |