Published online Apr 7, 2005. doi: 10.3748/wjg.v11.i13.1957
Revised: August 19, 2004
Accepted: October 6, 2004
Published online: April 7, 2005
AIM: We used isolated hepatocytes to investigate how different concentrations of ATP in the University of Wisconsin (UW) solution affected both cellular ATP content and cell viability during the cold storage and the rewarming step. The mechanism involved in ATP transport and accumulation in hypothermia was also determined.
METHODS: The cells were preserved up to 72 h in different conditions: UW solution without ATP (a-group), UW+5 mmol/L ATP (b-group), and UW+10 mmol/L ATP (c-group). The ATP content and the cell viability (LDH release) were determined during the cold storage and the rewarming step. In the groups a and c, the respiratory function of the cells at rewarming was studied. In addition, the cell volume of hepatocytes and the mechanism involved in ATP transport and accumulation were assessed. The extracellular degradation of exogenous nucleotides during transport experiments was investigated by a HPLC technique.
RESULTS: After three days of cold storage a loss of cellular ATP content was observed in hepatocytes preserved either without nucleotides (a-group) or with 5 mmol/L ATP (b-group). In contrast, 10 mmol/L ATP (c-group) was able to maintain a normal ATP cellular content, with only a 6% diminution after 72 h of cold storage. The respiratory function was significantly different in hepatocytes preserved with 10 mmol/L ATP than without ATP. No significant change was detected for the three groups in cellular volume during the cold storage. We also report that the time course accumulation of [3H]-ATP by cold stored hepatocytes is a rapid process that is completed after 180 s with linear dependence on the extracellular ATP concentration (linear fitting results in a slope of 0.5624±0.1179 mmol/L ATP intracell/mmol/L ATP extracell).
CONCLUSION: Our results show that, during hypothermic storage in UW solution, hepatocytes are permeable to ATP by a diffusive mechanism. Also, we found that it is ATP the main extracellular nucleotide available for transport and it is not the breakdown products.
- Citation: Mamprin ME, Vega F, Rodriguez JV. Adenosine 5'triphosphate transport and accumulation during the cold preservation of rat hepatocytes in University of Wisconsin solution. World J Gastroenterol 2005; 11(13): 1957-1964
- URL: https://www.wjgnet.com/1007-9327/full/v11/i13/1957.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i13.1957
Hypothermic preservation of organs in the University of Wisconsin (UW) solution is a widespread and well-accepted method of maintaining organs and isolated cells[1,2]. During the preservation process at low temperature (0°C), there is depletion of metabolites and of a time-dependent injury, which sensitizes cells to different forms of metabolic impairment, playing a negative role during transplantation[3]. We have used hepatocyte suspensions as the experimental model to study the effects of hypothermic storage in UW solution on liver cell metabolism and cell membrane properties[4]; also, for their possible application in cell transplantation and/or in bioartificial liver devices.
During the cold storage of entire organ or hepatocytes, there is a loss of adenine nucleotides that are hydrolyzed to adenosine, inosine, xanthine and hypoxanthine[5]. The mechanism of ischemic injury involves the loss of mitochondrial respiration resulting in ATP depletion and impairment of energy-dependent metabolic pathways and transport processes[6]. A number of experimental and clinical studies have shown the importance of the availability of energy substrates on liver viability, and a correlation between ATP synthesis of the preserved liver and survival after transplantation has been demonstrated[7]. Thus, it would be beneficial to develop new strategies directed to maintain the hepatocyte ATP level during cold storage and rewarming. Recently, Neveux et al[8], demonstrated that liposomally entrapped ATP supplied during the hypothermic preservation represents an effective means to improve liver graft energy state and function. Tan et al[9] have reported the protective effect of exogenous ATP using a continuously hypothermic machine perfusion model. Experiments performed at physiological temperature showed that ATP can enter the cells without hydrolysis[10,11], in contrast with the general consensus that adenosine rather than ATP crosses the cell membrane. Based on this evidence, it was concluded that the release and uptake of ATP is a physiological process also present in tissues such as liver and kidney.
More recently, two studies[12,13] have reported the effect of temperature on the hepatic uptake of different solutes (histidine, carnosine, mannitol, raffinose, glutathione and adenosine) from the preservation solution used during the cold storage. We have previously demonstrated on a cellular model that the incorporation of ATP to the UW solution is more effective than adenosine to increase both the ATP content and the rate of glutathione synthesis during the rewarming step[14]. Since the mechanism involved in such experiment was not investigated, the aim of the present study was to (1) determine the mechanism involved in ATP transport and accumulation in isolated rat hepatocytes during cold preservation under different conditions, and (2) evaluate the impact of the different preservation conditions on cell function during the rewarming process. Understanding the mechanism involved in this process may be extremely useful in the development of new methodologies to improve cell preservation.
Raffinose, lactobionic acid, streptomycin, penicillin G, and glutathione were from ICN Biochemicals, other chemicals compounds were from Sigma-Aldrich. 3H2O (Spec. act: 1 mCi/g) and [carboxyl-14C] inulin (Spec. act: 2.6 mCi/g) were obtained from New England Nuclear and [2,5',8-3H] adenosine 5'-triphosphate, ammonium salt (80.4 mCi/mg) from Amersham Pharmacia Biotech, England.
Male Wistar rats weighing 250-300 g were used in all experiments. Rats were allowed access to 5800 Formula Diet (LabDiet®, USA) and water ad libitum freely, prior to the experiment and received care in compliance with international regulations. The National Council Committee approved animal protocols.
The animals were anesthetized with an intraperitoneal injection of sodium thiopental (70 mg/kg). Hepatocytes were isolated by collagenase perfusion using the procedure described by Seglen[15] and modified by us[16]. The cell viability was tested by the exclusion of 0.4% Trypan blue stain (TBE) in phosphate-buffered saline. Over 500 million cells were obtained per liver and preparations with a TBE larger than 90% were considered suitable for the experiments.
Isolated hepatocytes were rinsed twice and resuspended in freshly prepared cold (0 °C) UW solution. The composition of the modified UW solution[4] was as follows: 100 mmol/L lactobionic acid, 25 mmol/L KH2PO4, 5 mmol/L MgSO4, 30 mmol/L raffinose, 3 mmol/L GSH, 1 mmol/L allopurinol, 5% polyethylene glycol (MW: 8000), 15 mmol/L glycine, 0.25 mg/mL streptomycin and 10 UI/mL penicillin G; pH 7.40. The solution was bubbled with 100% N2 for 15 min at 0 °C before use. Hepatocytes (75×106 cells in 30 mL UW solution) were allowed to settle to the bottom of the 50-mL screw cup polycarbonate tubes and left undisturbed at 0 °C up to 72 h. One of the stored suspensions was removed daily, and used to estimate the time course changes of the viability assessed by the lactate dehydrogenase (LDH) release and morphological aspects.
After 72 h of cold storage, the hepatocytes were washed twice with a rinse solution developed in our laboratory[16] and sedimented (50 g, 3 min) in warm Krebs-Henseleit resuspension (KHR) media. The KHR composition was 114 mmol/L NaCl, 25 mmol/L NaHCO3, 4.8 mmol/L KCl, 1.5 mmol/L CaCl2, 10 mmol/L hepes, 5 mmol/L fructose, 5 mmol/L glucose, 1 mmol/L allopurinol, 3 mmol/L glycine and 1% BSA; pH 7.20. The hepatocytes were subsequently incubated (120 min, 37 °C, 2-3×106 cells/mL) in KHR media under carbogen atmosphere in a Dubnoff metabolic shaker.
There were two parts of the study:
Part I The purpose of part I was to evaluate the effects of ATP addition in the UW solution on the ATP content and viability of isolated hepatocytes. The cells were preserved up to 72 h in different conditions: UW solution without ATP (a-group), UW+5 mmol/L ATP (b-group), and UW+10 mmol/L ATP (c-group). We determined the ATP content and the viability of the cells during the cold storage and during the rewarming step (LDH release). Also, for the groups a and c, we studied the respiratory function of the cells after 72 h of cold storage.
Part II The purpose of the part II of the study was to determine the mechanism involved in ATP transport and accumulation in isolated hepatocytes during cold preservation. To perform this, the following experiments were realized.
Freshly isolated hepatocytes were resuspended in UW solution (transport medium). The transport of ATP was carried out at 0 °C by addition of transport medium containing a constant concentration of [3H] ATP (3.13 μCi/mL) and variable concentrations of unlabelled ATP (to obtain a final concentration of 0.5, 1.0, 2.5, 5.0 and 10.0 mmol/L) to 1 mL of cell suspension. Cells (2.5×106 cells/mL) were maintained at 0 °C and transport was initiated by addition of 100 μL of transport medium. After incubation (10 min - 0 °C), the cell suspension was centrifuged (13000 g - 30 s), the cell-free supernatant was poured off and the residual medium was carefully removed from the pellet by aspiration with an 18 G needle connected to a vacuum pump. Radioactivity in the cell pellet and supernatant was determined by dissolving both in liquid scintillation cocktail (Optiphase Hisafe 3, Wallac, Finland) and counted in an LKB Wallac liquid scintillation system. ATP transport was measured in triplicate for each determination. Non-specific binding of label to the cells was determined as follows: 1 mL of hepatocyte suspension in UW was incubated at 0 °C with 100 μL of transport medium. After 10 min the cells were centrifuged, the supernatant was discarded and the cellular pellet lysate with 1 mL of distillate water was vigorously stirred, centrifuged and the supernatant was discarded (this procedure was repeated three times). Then, the resultant pellet was dissolved in liquid scintillation medium and counted as above.
The time course of ATP accumulation into hepatocytes was determined in transport medium. Extracellular ATP concentration of 10 mmol/L was chosen because it was more effective than other concentrations to enhance cellular ATP content (see below) without any loss in cell viability.
One milliliter of hepatocyte suspension (2.5×106 cells/mL) was incubated 15, 30, 45, 60, 90, 180, 300, 600 and 1200 s with 100 μL of the transport medium containing [3H] ATP (4.1 μCi/mL) and 10 mmol/L ATP. After incubation at 0 °C for the required time, uptake was terminated by rapid centrifugation (13000 g - 30 s). Pellet and supernatant were immediately separated and the pelleted hepatocytes were dissolved in liquid scintillation cocktail and the radioactivity counted as above.
We analyzed the medium in which the cells were cold stored to investigate the occurrence of extracellular degradation of exogenous ATP during transport experiments and the time course of ATP accumulation in hypothermia. The cells were incubated in different conditions: UW solution without ATP (a-group), UW+10 mmol/L ATP (b-group) and a third group used as control (d-group) where the UW solution+10 mmol/L ATP were cold preserved without cells. Then, we determined the extracellular concentration of high-energy nucleotides ATP, ADP and AMP after 3 and 60 min of cold storage.
The concentration of ATP, ADP and AMP in UW solution was determined by an HPLC technique. Briefly, the samples were centrifuged at 12000 g, 3 min and the supernatant fractions were filtered through a 0.2 μmol/L PES filter (Nalge, cat. 0001801320). A 100 μL aliquot of the filtered supernatant was injected into an HPLC system (loop 20 μL), consisting of a Gilson 307 pump, a guard column (Phenomenex - AJO 4287), a Nucleosil 5 μ ODS (C18) column (250 mm×3.2 mm) (Phenomenex - 00G-0323-R0) and a Gilson 151 UV-VIS detector set at 254 nm. The samples were eluted with a mobile phase of 13% acetonitrile: 87% buffer (0.09 mol/L potassium phosphate, 5.0 mmol/L tetrabutyl ammonium hydrogen sulfate) (v/v), pH 6.00 at a flow rate of 0.5 mL/min, resulting in a typical retention time of 4.74±0.02 min for AMP, of 6.73±0.06 min for ADP and of 10.60±0.33 min for ATP, n = 10. The peaks were identified and quantified using standards (ICN, Biochemicals, USA). The data were analyzed and processed using the 712 HPLC Control Software package from Gilson (Villiers - Le - Bel, France).
Total water content was expressed as μL H2O/106 cell and performed in quadruplicated for each experiment to produce a mean value. Ten microliters of hepatocyte suspension (2.5×106 cells/mL) in UW solution were incubated with 3H2O (1 μCi/mL) and [carboxyl-14C] inulin (0.5 μCi/mL) plus 0.2 mg/mL inulin for 20 min at 0 °C. The total water content was calculated for the following experimental groups: UW solution without ATP and UW added with 0.5, 1.0, 2.5, 5.0 and 10.0 mmol/L ATP. For each group, cells were separated from medium by centrifugation (13000 g - 30 s). After separation, samples of sedimented cells and medium were dissolved in liquid scintillation cocktail and the radioactivity counted. Intracellular water space was calculated from total cell water subtracting the trapped extracellular fluid (14C-inulin space) as previously described[17].
LDH release The activity of LDH in cell suspension (total activity) and in supernatant (extracellular LDH) was determined as described previously[4]. Results were expressed as the percentage of total enzyme activity in the extracellular medium.
ATP assay Hepatocytes (2.5×106 cells/mL) were separated from the incubation medium by centrifugation (13000 g - 30 s) and the cell pellet was deproteinized by the addition of 500 µL of cold 3% HClO4. After centrifugation, the protein-free supernatant was neutralized with K2CO3 and cell ATP contents were measured using the luciferase–luciferin assay as described by the manufacturer of the commercial assay kit (Sigma, FL-AA). The level of photoluminescence was quantified using a LKB Wallac Luminometer. ATP quantification was determined by comparison to a standard ATP curve.
Respiratory function of cold stored hepatocytes Freshly hepatocytes (controls) and cold stored cells in UW solution without ATP (a-group) and with 10 mmol/L ATP (c-group) were incubated in KHR medium at 37 °C in a Dubnoff metabolic bath under carbogen atmosphere with continuous shaking for 120 min. Mitochondria function was then assessed measuring respiratory activity of the cells. Values were calculated per 106 cells in the sample. At different intervals (0, 60 and 120 min) aliquots of cell suspensions containing 2×106 cells were centrifuged and resuspended in 100 μL of respiration medium and added to a thermostatized oxygen electrode chamber containing 1.9 mL of respiration medium (Krebs-Henseleit media (KH), pH 7.40, containing hepes 10 mmol/L, pyruvate 2 mmol/L, (without NaHCO3) at 36 °C. The oxygen content of respiration medium equilibrated with air was determined using the method of Robinson[18] and was 0.524±0.024, n = 10 mmol/mL at 36 °C. Oxygen consumption was measured using a Clark-type oxygen electrode (YSI 5300, Yellow Spring, OH, USA) under the following conditions[19,20]:
(1) Basal respiration or endogenous respiration (Vend): rate of oxygen consumption in the incubation medium. A linear rate of oxygen consumption was obtained in about 2 min, after which the rate of oxygen uptake was recorded and calculated over a 2-min period.
(2) Succinate-stimulated respiration (Vsucc): This substrate slowly crosses the plasma membrane of normal hepatocytes and causes little stimulation of the endogenous respiration rate. However, in cells with compromised membrane integrity, succinate may more easily pass into the cells[19] and produce a marked stimulation of respiration greater than twofold, as shown in chemically permeabilized cells[21]. After the determination of basal respiration, succinate (10 μL) was added to the chamber (2 mmol/L final concentration) and the rate was recorded over 2 min. The total measurement time including the succinate test was 6 min. Oxygen consumption measurements were made on at least three independent samples for each experimental condition.
Results are presented as mean±SD and the number of preparations analyzed was three or more, as indicated in each figure. The statistical significance of the differences between values was assessed by one-way or multifactor analysis of variance followed by Scheffe’s multiple range test, as it was convenient. A P<0.05 was considered to be statistically significant (Statgraphics, Statistical Graphics System, USA).
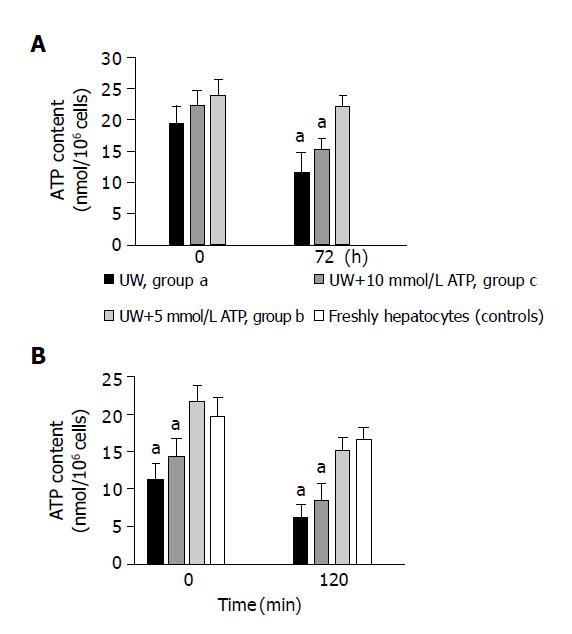
Figure 1A shows the ATP content of preserved hepatocytes from groups (a), (b) and (c) at time 0 and after 72 h of preservation. All groups showed a similar value of ATP content at time zero. After 72 h of cold storage a significantly (P<0.05) higher ATP cell content was found in cells preserved in the presence of 10 mmol/L ATP (c-group) (22.11±1.83 nmoL ATP/106 cells), than in cells from a-group (11.52±3.12) and b-group (15.12±1.45) (n = 6 preparations).
To exclude the possibility that the higher concentration of ATP seen in the c-group was artifactual due to ATP trapped in the extracellular media of sedimented hepatocytes, we prepared experiments in which the extracellular water was determined (0.13±0.04 μL H2O/106 cells) (n = 6 preparations). The volume of extracellular water containing 10 mmol/L ATP would increase the cellular ATP content by 1.30 nmoL ATP/106 cells, a value at least 10 times lower than that was found.
Figure 1B shows the ATP content in freshly isolated and in cold stored hepatocytes from groups (a), (b) and (c) 0 and at 120 min after rewarming. At time 0, controls and c-group showed a similar value of ATP content 19.64±2.44 nmoL ATP/106 cells (n = 5) and 21.65±1.93 nmoL ATP/106 cells (n = 4) respectively. In cells from group (a) and (b), the ATP content was significantly decreased by the cold storage to 42.3±3.8% and 27.1±3.9% of controls respectively (n = 4). After 120 min of rewarming a significantly (P<0.05) higher ATP cell content was found in c-group (15.12±1.73 nmoL ATP/106 cells) than both in cells from a-group (6.98±2.34) and b-group (7.84±0.95) (n = 4). The ATP content in group (c) was similar to that observed in freshly isolated hepatocytes (16.55±1.55) (n = 4).
Viability of suspensions of hepatocytes stored in different modified UW solutions was assessed by LDH release. Seventy-two hours after preservation, no statistical difference was found among groups a, b and c. LDH release was calculated as a mean between these three preserved groups at each time. At the start of the experiments (time = 0), the LDH release was 0.31±0.17% (n = 12) of total cellular activity. Hepatocyte viability was well maintained during cold storage of cell suspensions for up to 72 h (LDH released at 72 h:3.13±0.25%) (n = 9). Preliminary experiments indicated that on the basis of cellular LDH release (14) 72 h of cold storage is the time limit in cold preservation. On the other hand, in our first investigations we preserved hepatocytes in UW with 15 mmol/L ATP. In this case the cells show a dramatic decrease in the viability and in the ATP content after 72 h of cold storage. The LDH release at 72 h was 6.55±0.43%, and the ATP content was 6.44±1.92 nmoL ATP/106 cells (n = 4). These values were statistically different in comparison to the other groups (P<0.05). The cell damage observed at high ATP concentration suggests the triggering of some mechanism that dramatically affects both ATP and cell viability. Some reports[22,23] have shown that extracellular ATP can induce cell death (apoptosis and necrosis) in isolated hepatocytes. The mechanism of cell death in our experimental conditions (UW+15 mmol/L ATP, 0 °C) was not studied in this work and in view of this “toxic effects” the following experiments were done with a maximal concentration of ATP of 10 mmol/L.
The LDH release was also used to assess hepatocyte injury during rewarming studies. After 120 min of rewarming, the LDH release (%) was significantly lower (P<0.05) for cells from c-group (13.11±1.62) in comparison with cells from a-group (19.87±1.96) and from b-group (n = 3). No differences in LDH release between the cells preserved with 10 mmol/L ATP and freshly isolated hepatocytes (12.16±1.39) (n = 3) were observed.
Table 1 shows the indices of the respiration activity in freshly isolated hepatocytes (controls) and hepatocytes after cold storage in UW solution (a-group) or in UW with 10 mmol/L ATP (c-group) with subsequent normothermic incubation for 120 min.
| Indices | Freshly Isolated Hepatocytes (Controls) Incubated in KHR | Cold preserved Hepatocytes 72 h in UW without ATP (a-group).Incubated in KHR | Cold preserved Hepatocytes 72 h in UW with 10 mM ATP (c-group).Incubated in KHR | ||||||
| 0 min | 60 min | 120 min | 0 min | 60 min | 120 min | 0 min | 60 min | 120 min | |
| Endogenous respiration(Vend) | 336 | 304 | 285a | 3210 | 175bd | 124ade | 353 | 214bd | 213ad |
| Respiration Stimulated by succinate(Vsucc) | 4910 | 5013 | 5015 | 599 | 5317 | 578 | 522 | 509 | 598 |
| Vsucc/Vend | 1.480.10 | 1.670.23 | 1.770.27a | 1.940.45 | 3.410.81bcdf | 4.420.96ad | 1.490.10 | 2.100.30bdf | 2.790.46ad |
Freshly isolated hepatocytes showed a high level of Vend (33±6 nmoL O2/min/106 cells) and Vsucc (49±10 nmol O2/min/106 cells) (n = 3). Values which were maintained during a period of 60 min of normothermic incubation only slightly reduced after 120 min. The Vsucc/Vend ratio at time 0 of incubation was 1.48±0.1 (n = 3) and statistically increased over a period of 120 min of normothermic incubation, in line with previous reports[19]. Table 1 shows that cold storage of hepatocytes resulted in a significant decrease in Vend and an increase (P<0.05) in Vsucc/Vend ratio after 60 and 120 min of rewarming. Although we found significant differences between the preserved cells and freshly isolated hepatocytes, it is important to note that the cells preserved in the presence of 10 mmol/L ATP (c-group) shown best indices of respiration activity as compared with the a-group (P<0.05).
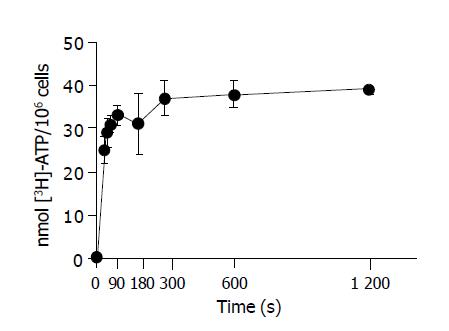
Cells were resuspended in UW at 0 °C. Incubation of hepatocytes with 10 mmol/L [3H] ATP resulted in the net accumulation of [3H] ATP into hepatocytes over time. The incorporation of label into cells was corrected for the trapping medium ATP at every time by the [14C] inulin space associated with the cells, which remained constant during the incubation period. Figure 2 shows net ATP accumulation by hepatocytes, corrected for trapping and non-specific binding to cells. The accumulation of [3H] ATP under conditions of cold storage in UW was rapid and completed after 180 s.
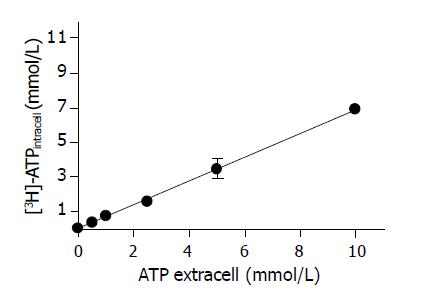
The evaluation of the kinetic of ATP accumulation was done at 10 min of incubation. Figure 3 shows the relationship between ATP cellular accumulation and the extracellular ATP concentration. The accumulation of [3H] ATP by cold-stored hepatocytes was a non-saturable process related to the extracellular ATP concentration. By plotting the extracellular vs intracellular ATP concentration, a linear fitting was obtained (slope of 0.5624±0.1179 mmol/L ATP intracell/mmol/L ATP extracell) n = 24 (r = 0.9896), indicating that the hepatocyte ATP accumulation at 0 °C of temperature occurs by a diffusion mechanism.
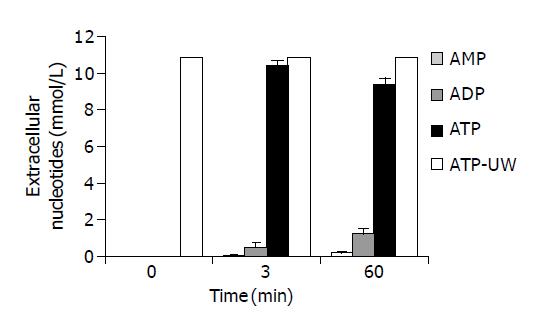
Figure 4 shows the time course evolution of extracellular ATP and nucleotides measured over 60 min of cold storage. The white bar shows that UW solution+10 mmol/L ATP without cells maintain the nucleotide level unchanged during the 60 min of cold storage. Three minutes after the addition of 10 mmol/L ATP to cell suspension, ATP was 95.5% of the total extracellular nucleotides, ADP 4.1% and AMP 0.5%. After 60 min, the exogenous ATP was catabolized probably by ecto ATPases around 87% of the total extracellular nucleotides and ADP and AMP rises to 11.4% and 1.6%, respectively.
In order to rule out the possibility that the higher content of ATP found in hepatocytes cold stored in UW+ATP was due to changes in cell volume, we examined the effects of incubating cells in UW containing 10 mmol/L ATP on the cell volume. Freshly isolated hepatocytes resuspended in Krebs-Henseleit solution was used as controls. The intracellular water space of control cells was 11.94±2.54 μL H2O/106 cells (n = 6 preparations), while cold storage in UW solution without ATP reduced cell volume to 5.47±0.27 μL H2O/106 cells (n = 6 preparations). Cells stored in UW+10 mmol/L ATP showed a volume of 6.10±0.34 μL H2O/106 cells (n = 4 preparations). Since no statistically significant change in cell volume was detected by the addition of ATP to UW solution, the higher concentration of ATP in these hepatocytes was not due to changes in cellular volume. Reported in Table 2, the water space distribution of cold-stored hepatocytes was not modified by the addition of ATP to UW solution at final concentrations of 0.5, 1, 2.5, 5.0, and 10.0 mmol/L. In our experiments, the cell volume control is similar to that previously reported[17] and hepatocyte shrinkage appeared in UW preservation as described by Crenesse et al[24].
| Extracellular concentration of | H2Ointracellular (mL/106 cells) | H2Oextracellular (mL/106 cells) |
| ATP (mmol/L) | ||
| – | 5.47±0.27 | 0.10±0.03 |
| 0.5 | 6.35±0.91 | 0.11±0.03 |
| 1 | 5.43±0.63 | 0.09±0.01 |
| 2.5 | 5.77±0.87 | 0.12±0.04 |
| 5 | 5.48±0.33 | 0.11±0.04 |
| 10.0 | 6.10±0.34 | 0.13±0.03 |
In this study we have examined the transport and accumulation of ATP during cold preservation of rat hepatocytes in UW solution. Our results are in line with those of Vreugdenhil and Southard[25,26] and shows that the cellular concentration of ATP decreases during cold storage in the absence of added nucleotides. We have previously showed[14] that, under hypothermic conditions, the addition of extracellular ATP was most effective increasing cellular ATP during the rewarming step than adenosine. Moreover, the cells preserved in the presence of 5 mmol/L adenosine show a loss of GSH synthesis during rewarming and this phenomenon could well be a consequence of the effects of ATP loss produced by cold storage.
This study was designed to evaluate the effect of ATP addition to the UW solution on the hepatocyte ATP content during cold storage and rewarming. Figure 1A shows how the concentration of ATP in the UW solution affected the cellular ATP content during 72 h of preservation. After three days of cold storage, we observed a loss of cellular ATP in the hepatocytes preserved without nucleosides (a-group) or preserved with 5 mmol/L ATP (b-group). However, when 10 mmol/L ATP was added to the UW solution, the intracellular concentration of ATP rose and this fact was unrelated to changes in the cellular volume (Table 2). We also found that hepatocytes are permeable to ATP during hypothermic storage in UW. The viability tests (LDH release and microscopic observation) did not reveal any damage to cell membrane when the cells were preserved with 5 or 10 mmol/L ATP. Since cellular morphology and membrane integrity are only crude indices of functional survival after hypothermia, hepatocyte respiration was used as a more stringent metabolic test[27]. The cellular oxygen consumption was determined after 72 h of cold preservation to allow the expression of any latent damage during reperfusion. When the cells were cold stored in either UW or UW+10 mmol/L ATP for 72 h and rewarmed (37 °C), respiration was reduced with a respiration activity statistically lower than that in freshly isolated rat hepatocytes, suggesting severe impairment of oxidative metabolism. This is likely to be a multifactorial damage resulting from several factors including both depletion of cell solutes and disruption of mitochondrial architecture[28]. An interesting observation was that the decrease in respiratory function was greater for cells preserved in UW solution without ATP addition (a-group) than that in cells preserved with the presence of 10 mmol/L ATP. Under the latter condition the hepatocytes showed an elevated oxygen uptake and better indices of respiratory activities, possible due to a high ATP/ADP ratio and energy charge. In addition, the succinate exposure test was even more predictive of a progressive damage. After 72 h of cold storage in UW solution without ATP, the stimulation of respiration induced by succinate reached levels close to those in permeabilized cells which have lost metabolic control.
The conditions we used to add ATP during the experimental protocols are not fully comparable to those used previously[9,29-31] where the nucleotide was added as MgCl2: ATP complex. In our study, the addition of ATP was made directly into the UW solution. This choice was based on a double reasoning:
(1) the UW solution contains 5 mmol/L Mg++ and (2) previous studies from our group[14] showed that the incorporation of ATP in the UW solution increased the ATP content of cold-stored hepatocytes.
In order to assess the ATP uptake from the cells, radio-labeled ATP was used and found that the accumulation was a rapid and linear process (Figure 2). The fact that the mechanism involved in the accumulation of [3H] ATP by hepatocytes during cold storage is rapid and nonsaturable process, linearly dependent on by the extracellular ATP concentration, strongly suggests diffusion (Figure 3). Former studies have demonstrated increased intracellular purine nucleotides after treatment with exogenous purines. These experiments were made at normothermic temperatures[29-31] in which the occurrence of rapid extracellular degradation of exogenous nucleotides question whether nucleotide uptake could be involved. The investigations of Chaudry and Clemens[29] indicated that freshly isolated hepatocytes do show ATP uptake component in, which is independent of prior metabolism to adenosine. In order to further substantiate the ATP metabolism we investigated the occurrence of extracellular degradation of exogenous ATP during transport experiments and demonstrated that ATP is the main nucleotide available to be transported into the cells and not its breakdown products such as ADP or AMP. The ecto ATPase activity in hepatocytes was significant only around 60 min of cold storage.
Previous evidence showed that an increase in permeability to solutes, normally excluded by the bilayer, occurs following the temperature-induced changes in the cell membrane. Therefore, at 0-4 °C, solutes contained in the solution would enter the cell by diffusion[12,13]. On the other hand, studies performed in the whole organ showed the same difference from the cellular models, for example, in the kinetic of ion exchange[32]. This conclusion is also supported by the fact that, in the whole-organ model, the extracellular space is much smaller than the intracellular space, in contrast to cellular models where the extracellular space is almost infinite facilitating a continuous transmembrane ion gradient[33].
Our results indicate the improvements that can be made to currently used hepatocyte preservation solutions in respect to maintain a high-energy phosphate compound as ATP at the cellular level. In this way, ATP levels must be generated in sufficient quantity to restore critical cellular processes, such as ion exchange channel gradients across plasma and mitochondrial membranes, to promote protein synthesis and to supply the urea cycle[12].
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 986] [Cited by in RCA: 952] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 2. | Sandker GW, Slooff MJ, Groothuis GM. Drug transport, viability and morphology of isolated rat hepatocytes preserved for 24 hours in University of Wisconsin solution. Biochem Pharmacol. 1992;43:1479-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Southard JH, van Gulik TM, Ametani MS, Vreugdenhil PK, Lindell SL, Pienaar BL, Belzer FO. Important components of the UW solution. Transplantation. 1990;49:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 209] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Rodríguez JV, Mamprin ME, Mediavilla MG, Guibert EE. Glutathione movements during cold preservation of rat hepatocytes. Cryobiology. 1998;36:236-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Fuller BJ. Effects of cooling on mammalian cells. Clinical applications of Cryobiology. Boca Raton: CRC Press 1991; 1-21. |
| 6. | Bilzer M, Gerbes AL. Preservation injury of the liver: mechanisms and novel therapeutic strategies. J Hepatol. 2000;32:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 7. | González FX, Rimola A, Grande L, Antolin M, Garcia-Valdecasas JC, Fuster J, Lacy AM, Cugat E, Visa J, Rodés J. Predictive factors of early postoperative graft function in human liver transplantation. Hepatology. 1994;20:565-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 205] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Neveux N, De Bandt JP, Fattal E, Hannoun L, Poupon R, Chaumeil JC, Delattre J, Cynober LA. Cold preservation injury in rat liver: effect of liposomally-entrapped adenosine triphosphate. J Hepatol. 2000;33:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Tan XD, Egami H, Wang FS, Ogawa M. Protective effect of exogenous adenosine triphosphate on hypothermically preserved rat liver. World J Gastroenterol. 2004;10:871-874. [PubMed] |
| 10. | Chaudry IH, Sayeed MM, Baue AE. Uptake of ATP by liver and kidney in vitro. Can J Physiol Pharmacol. 1976;54:742-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Chaudry IH. Does ATP cross the cell plasma membrane. Yale J Biol Med. 1982;55:1-10. [PubMed] |
| 12. | So PW, Fuller BJ. Hepatic uptake of solutes from the preservation solution during hypothermic storage: a (1)H NMR study in rat liver. Cryobiology. 2001;42:307-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Schilling M, Tian YH, Büchler MW. Effect of temperature on hepatic and renal uptake of components from University of Wisconsin solution. Transplantation. 1998;65:989-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Mamprin ME, Guibert EE, Rodriguez JV. Glutathione synthesis during the rewarming of rat hepatocytes preserved in the University of Wisconsin solution. Cryobiology. 2001;43:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3785] [Cited by in RCA: 3877] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 16. | Mamprin ME, Guibert EE, Rodriguez JV. Glutathione content during the rinsing and rewarming process of rat hepatocytes preserved in University of Wisconsin solution. Cryobiology. 2000;40:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Fariss MW, Brown MK, Schmitz JA, Reed DJ. Mechanism of chemical-induced toxicity. I. Use of a rapid centrifugation technique for the separation of viable and nonviable hepatocytes. Toxicol Appl Pharmacol. 1985;79:283-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Robinson J, Cooper JM. Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem. 1970;33:390-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 295] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Kravchenko LP, Petrenko AY, Somov AY, Grischenko VI, Fuller BJ. Respiratory activity of isolated rat hepatocytes following cold storage and subsequent rewarming: a comparison of sucrose-based and University of Wisconsin solutions. Cryobiology. 2001;42:218-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Eaton DL, Klaassen CD. Carrier-mediated transport of ouabain in isolated hepatocytes. J Pharmacol Exp Ther. 1978;205:480-488. [PubMed] |
| 21. | Mapes JP, Harris RA. On the oxidation of succinate by parenchymal cells isolated from rat liver. FEBS Lett. 1975;51:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Richter C, Schweizer M, Cossarizza A, Franceschi C. Control of apoptosis by the cellular ATP level. FEBS Lett. 1996;378:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 351] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 23. | Leist M, Single B, Castoldi AF, Kühnle S, Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med. 1997;185:1481-1486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1469] [Cited by in RCA: 1427] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 24. | Crenesse D, Fossat B, Craffa F, Chaland P, Porthe-Nibelle J, Poiree JC, Gugenheim J. Potassium uptake and water content in hepatocytes isolated from rat livers preserved in Euro-Collins and UW solutions and after transplantation. Cryobiology. 1994;31:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Vreugdhenhil PK, Belzer FO, Southard JH. Adenine stimulation of adenosine triphosphate synthesis in cold-stored hepatocytes. Transplantation. 1991;51:909-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Kim JS, Southard JH. Effect of liver preservation on hepatocyte calcium and ATP regeneration. Transplant Proc. 1997;29:3447-3448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Kantrow SP, Taylor DE, Carraway MS, Piantadosi CA. Oxidative metabolism in rat hepatocytes and mitochondria during sepsis. Arch Biochem Biophys. 1997;345:278-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Sammut IA, Thorniley MS, Simpkin S, Fuller BJ, Bates TE, Green CJ. Impairment of hepatic mitochondrial respiratory function following storage and orthotopic transplantation of rat livers. Cryobiology. 1998;36:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Chaudry IH, Clemens MG. Dissociation between ATP and adenosine uptake by hepatocytes. Fed Proc. 1982;41:1156. |
| 30. | Weinberg JM, Humes HD. Increases of cell ATP produced by exogenous adenine nucleotides in isolated rabbit kidney tubules. Am J Physiol. 1986;250:F720-F733. [PubMed] |
| 31. | Weinberg JM, Davis JA, Lawton A, Abarzua M. Modulation of cell nucleotide levels of isolated kidney tubules. Am J Physiol. 1988;254:F311-F322. [PubMed] |
| 32. | Tian Yh, Fukuda C, Schilling MK. Interstitial accumulation of Na+ and K+ during flush-out and cold storage of rat livers: implications for graft survival. Hepatology. 1998;28:1327-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Klöppel K, Gerlach J, Neuhaus P. The electrolyte composition of liver preservation solutions for hepatocytes in a model of in vitro preservation and reoxygenation. Langenbecks Arch Chir. 1994;379:210-217. [PubMed] |