INTRODUCTION
Cholestasis, extrahepatic or intrahepatic, is a common pathophysiological process in many human diseases leading to the accumulation of toxic bile salts within the liver[1-5]. It seems likely that the detergent action of bile salts is responsible for solubilization of plasma membranes and cell death, which in turn may lead to oxidative stress, oxidation of reduced glutathione (GSH), and lipid peroxidation[6]. There is growing evidence suggesting that considerable impairment of oxidative stress regulation may play an important role in cholestatic liver injury[7-11]. Acute bile-duct obstruction is characterized by increased lipid peroxidation and by marked decline in reduced GSH, a major cellular antioxidant[12]. It is known that bile-duct ligation (BDL) results in a shift in the oxidant/prooxidant balance in favor of increased free radical activity[12,13]. Enhanced production of reactive oxygen intermediates augments lipid peroxidation by disturbing oxidant-antioxidant balance in hepatic mitochondrial fraction. The damage pattern of free radicals interfering with hepatocytes suggests peroxynitrite-mediated liver injury[14]. As a reactive-free radical, NO mediates the cytotoxicity caused by activated neutrophils and macrophages in the inflammatory response[15,16].
In attempting to limit the oxidative damage, a number of antioxidants have been tested in experimental bile-duct obstruction models[6,7,12]. It has been proposed that antioxidants, which maintain the concentration of reduced GSH, may restore the cellular defense mechanism and block lipid peroxidation[10]. Melatonin has been proved to have the greatest impact not only on oxidative stress, but also on systems of defense against free radicals, restoring the oxidative balance in treated experimental animals[10,12]. Administration of melatonin at pharmacological doses has been shown to decrease free radical formation and lead to a substantial recovery of the major antioxidant enzymes, thus limiting oxidative damage to the liver[12]. Recent evidences have shown that melatonin has protective effects on hepatic injury after extrahepatic BDL in rats[10,11,17].
The aims of this study were first to investigate the role of oxidative injury and the effect of exogenous melatonin administration on liver damage induced by BDL, and second, to evaluate the role of NO in oxidative injury. Hepatic oxidative stress markers were evaluated by changes in the amount of lipid peroxides, measured as MDA and GSH. Since NO measurement is difficult in biological specimens, tissue nitrite (NO2) and nitrate (NO3) were estimated as an index of NO production. Additionally, a detailed histopathological examination using a histological scoring system was performed. To our knowledge, there has been only one study revealing histological results about the effects of melatonin on experimental cholestatic liver injury in rats[17]. We suggest that NO contributes to oxidative stress and melatonin is a possible protective agent in biliary cholestasis and parenchymatous liver injury.
MATERIALS AND METHODS
Animals and experimental groups
Thirty-two adult male Sprague-Dawley rats weighing 250-290 g were used. Animals were housed under continuous observation in appropriate cages in a quiet temperature (21±2 °C) and humidity (60±5%)-controlled room in which a 12-12 h light-dark cycle was maintained. They were allowed free access to a commercial standard diet and water ad libitum. Rats were randomly assigned to four groups each containing eight rats as follows: sham operation (SO) (control), BDL, BDL+melatonin and BDL+vehicle. Sham-operated rats served as controls. Except in this group, biliary canals were ligated. Rats were fasted for 12 h before the operation, but were given water.
Animal experiments were performed in accordance with the guidelines for animal research from the National Institute of Health and were approved by the Committee of Animal Research at Inonu University, Malatya, Turkey.
Surgery procedure
Each rat was weighed and anesthetized by intraperitoneal administration of ketamin (70 mg/kg) and xylacaine (7 mg/kg). The abdomen was shaved and disinfected with 10% povidone iodine. Following a midline incision, the common bile duct was exposed and a double ligature with 3/0 silk was performed and the bile duct was sectioned between the ligatures. Abdominal muscles were closed with 3/0 silk and abdominal skin was closed with 2/0 silk. Sham surgery was identical to the ligation procedure, including locating and manipulating the common hepatic duct, except that the bile duct was not ligated or sectioned. Rats were maintained on the retrospective preoperative diet after surgery. Melatonin (Sigma Chemical Co., St. Louis, MO) 500 µg/(kg·d) was dissolved in ethanol and further diluted in saline (0.9 mL/L NaCl) to give final concentration of 1%. It was given intraperitoneally at 10.00 a.m. daily, beginning 1 d before the operation and continuing for seven successive days. BDL+vehicle group received equal volume (1 mL) of diluted ethanol in saline (0.9 mL/L NaCl). Rats were killed on the 8th d after the surgery. After the macroscopic findings were noted, the livers were promptly removed and were processed for histological and biochemical examination. The right lobe of the liver was divided into two pieces. The first samples were placed in 40 g/L formaldehyde for histopathological examination by light microscopy. The second piece of liver tissues was washed thrice with cold saline solution, placed into glass bottles, labeled, and stored in a deep freeze (-85 °C) for MDA, NO, and GSH analyses.
Histological examination
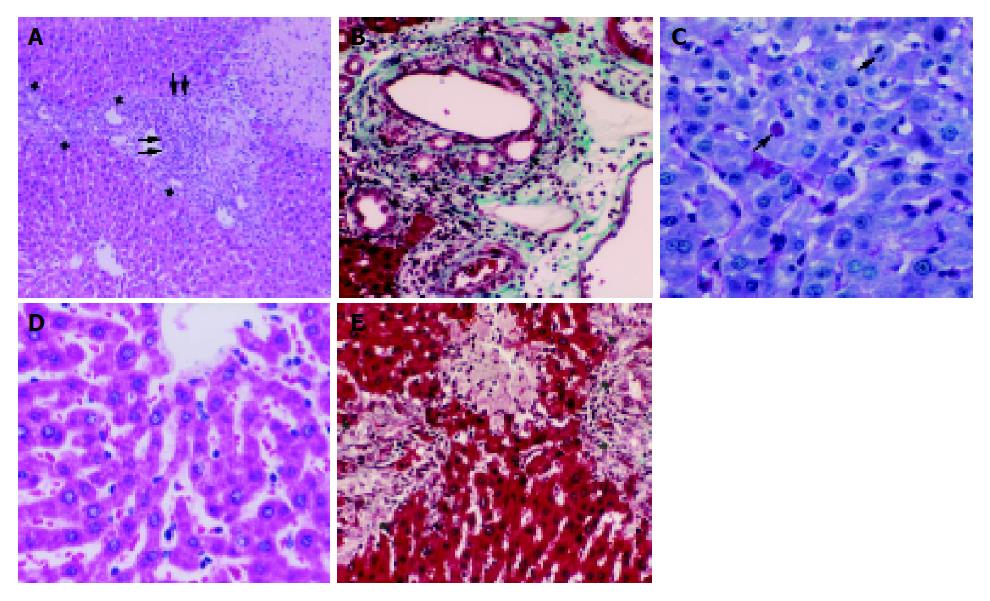
Liver tissues were fixed in 40 g/L formaldehyde and were embedded in paraffin. For histopathological evaluation, 4-mm slides were stained with hematoxylin-eosin, Masson’s trichrom, Periodic acid-Schiff (PAS), and Hall’s stain for bile. Sections were scored by an independent observer blinded to the experimental protocol. The following lesions were scored according to Modified Histological Activity Index (HAI):[18,19] portal inflammation, focal necrosis, confluent necrosis, piecemeal necrosis, apoptosis, focal inflammation and fibrosis. The number of biliary canals in five portal sites for each section was also noted.
Biochemical determination
Tissues were homogenized in four volumes of ice-cold Tris-HCl buffer (50 mmol/L, pH 7.4) using a homogenizer (IKA-Werke Ultra-Turrax T25 basic homogenizer, Germany) after cutting of the liver into small pieces. The malondialdehyde (MDA), nitric oxide (NO) and reduced GSH content of homogenates were determined spectrophotometrically.
Assessment of lipid peroxide formation Lipid peroxide was determined calorimetrically in a sample of the liver homogenates as thiobarbituric acid-reactive substances (TBA-RS) according to the method of Uchiyama and Mihara[20]. The absorbances of the formed colored product at 520 and 535 nm were measured and the results were expressed as the difference in absorbance at the two wavelengths (A535-520). MDA are expressed as micromole per gram wet hepatic tissue.
NO determination As NO measurement is very difficult in biological specimens, tissue nitrite (NO2) plus nitrate (NO3) concentrations were estimated as an index of NO production. The method for tissue NO2 plus NO3 levels based on the Griess reaction was used[21]. Samples were initially deproteinized with Somogy reagent[22]. Total nitrite (NOX) (NO2+NO3) was measured after conversion of nitrate to nitrite by copperized cadmium granules by a spectrophotometer at 545 nm. Results are expressed as nanomole per gram wet hepatic tissue.
Reduced glutathione determination Liver tissues were deproteinated by the addition of trichloroacetic acid. DTNB [5,5’-dithiobis(2-nitrobenzoic acid)] was added to supernatants cleared by centrifugation (10 min, 3000 r/min). The formation of 5-thio-2-nitrobenzoic acid, which is proportional to total GSH concentration, was monitored at 412 nm at 25 °C against reagent controls[23]. The level of GSH was determined from the standard curve with commercially available GSH (Sigma Chemical Co.). GSH are expressed as micromole per gram wet hepatic tissue.
Statistical analysis
The results were statistically analyzed by the Kruskal-Wallis H test. The differences between groups were evaluated by the Mann-Whitney U test followed by t test with Bonferroni correction when indicated. P<0.05 was considered significant. The results are expressed as the arithmetic mean±SE.
DISCUSSION
When a large bile duct is obstructed at any point in its course, several microscopic changes follow[24]. The morphological features of cholestasis depend on its severity, duration, and underlying cause[1]. The ducts themselves dilate and are filled with abundant bile[1,24]. Droplets of bile pigments can accumulate within hepatocytes[1]. Within a few days of the onset of obstruction, alterations are found in the portal tracts. The portal tracts become swollen as a result of edema of the connective tissue and infiltration by PNL, a classical acute inflammatory reaction attributed to the action of toxic bile salts reabsorbed from the bile by small bile ducts[2,24]. The bile stasis and backpressure due to obstruction induce proliferation of the duct epithelial cells and looping and reduplication of ducts, termed ‘bile duct proliferation’[1]. If the obstruction is not relieved, increasing fibrosis around ducts and extending between adjacent portal tracts develops[1,2,24]. Biliary obstruction leads to the death of hepatocytes due to bile accumulation[2,4]. Hepatic injury and cell death leads ultimately to liver fibrosis, cirrhosis, and cancer[2].
In the present study, bile duct proliferation in all groups compared to the controls was a result of the biliary obstruction. The number of bile ducts in five portal sites for each section did not show significant differences among the BDL, BDL+vehicle and BDL+melatonin groups (P>0.05) since obstruction was not relieved. Abdel-Aziz et al[25], observed extensive bile duct proliferation and formation of periportal fibrosis, with only slight inflammation and necrosis in their experimental model of extrahepatic cholestasis in rats. We have observed many histopathological features of cholestasis such as focal necrosis, confluent necrosis, piecemeal necrosis, focal and portal inflammation, bile duct proliferation and fibrosis in our experimental model. HAI scores of the BDL and BDL+vehicle groups were significantly higher than that of the SO group. Moreover, the obstructive jaundice was demonstrated by the coloration of the skin, peritoneum, and urine, and the dilatation of the common bile duct above the obstruction point. On the other hand, although bilirubin is one of the easiest pigments to be identified in standard HE sections, we could not observe it either in canaliculi or in bile ducts even though we used Hall’s stain for bile salts. Bile pigment accumulation within hepatocytes, canaliculi or bile ducts has been supposed to be one of the characteristic features of cholestasis[1]. As far as we know, in rodents the absence of bile salts after BDL has already been reported twice before in the studies of Prado et al[4], and Trauner and Boyer[26]. It is probably due to the induction of alternative detoxification and elimination pathways, which is very pronounced in rodents[26]. We think that bile salt accumulation may not accompany the other characteristic features of cholestasis in rodents.
Cell degeneration and death in cholestasis have been reported to be related to retention of toxic bile salts[2,4,6]. It seems that the detergent action of bile salts is responsible for solubilization of plasma membranes and cell death, which in turn may lead to oxidative stress[6]. It is shown that the acute infusion of toxic bile salts responsible for cholestasis induces zone 1 hepatocellular necrosis[5]. Padillo et al[27], have observed marked necrosis and apoptosis induced by cholestasis. In the present study, we observed numerous areas of coagulation necrosis and many apoptotic bodies in the BDL and BDL+vehicle groups. However, since we have not been able to identify accumulation of bile salts within parenchyma, we are suspicious about the direct, inducing role of the bile salts on the hepatocellular injury after BDL although, we cannot fully exclude the role of these products. A possible explanation is that an oxidative stress initiated by bile salts would make hepatocytes more susceptible to the toxic effects of small amount of bile salts, of which great amounts have been detoxified, that would not ordinarily injure the hepatic cells.
We observed many mitotic figures both in hepatocytes and in ductular epithelial cells. Mitosis and fibrosis within the parenchyma are the signs of wound repair. Increased cell proliferation and increased secretion of collagen and the other matrix proteins are important compensatory mechanisms for the repair of the injured tissues[28]. Mitosis in the ductular epithelium is the evidence of bile duct proliferation. The bile stasis and backpressure induce proliferation of the duct epithelial cells[1]. Bülbüller et al [17] observed mitotic figures in parenchyma and proliferation of biliary canals 7 d after experimental biliary canal obstruction. After bile-duct obstruction, the deposition of connective tissue elements and formation of ductular proliferates rapidly set in[29]. In our study, fibrous expansion of most portal areas was observed but portal to portal bridging was not present. So, HAI score for fibrosis was relatively low.
In obstructive jaundice, glucose metabolism undergo changes, the most common being the development of glucose intolerance[17,30]. Recent studies have reported a decrease in serum glucose values[17] and hepatic glycogen content[31,32]. We have observed nearly a total loss in the amount of glycogen in hepatocytes. Loss of appetite, reduction of oral food intake, reduced glycogen synthesis, possibly related to endotoxinemia, may probably be the causes of decrease in the hepatic glycogen content[17,29,32].
Many studies have reported that there is a correlation between the intensity of biliary tract obstruction and increased free radical generation, as well as a direct correlation between the level of oxidative stress and the biochemical markers of liver injury[6-11,14]. It has been well established that deleterious accumulation of lipid peroxides is correlated with marked impairment of soluble antioxidant defense mechanisms and cell necrosis[5,9,14,33]. Acute BDL is associated with an increase in MDA levels and a decrease in GSH levels both in plasma and in tissue[9,10,17,34-37]. Cruz et al[37], have reported an increase in MDA and a decrease in GSH levels in renal tissue after biliary obstruction. As observed in previous studies, biliary tract obstruction was accompanied by increased levels of lipid peroxidation and by the depletion of GSH, a ubiquitous antioxidant, in hepatic tissue in our study. On the other hand, there is increasing evidence that the enhanced production of reactive oxygen intermediates augments lipid peroxidation by disturbing the oxidant-antioxidant balance in hepatocytes. Since the levels of NOX in the liver were significantly higher in the BDL and BDL+vehicle groups than in the SO group (P<0.05), we suggest that NO, probably via its derivative peroxynitrite, may contribute to oxidative damage. Engin et al[14], reported a significant increase in plasma NO2, NO3 and NOX concentrations in inbred albino guinea pigs 24 h after common BDL.
A number of antioxidants have been tested in experimental bile-duct obstruction models in the attempt to limit the oxidative damage[7,12]. Administration of melatonin at pharmacological doses has been shown to decrease free radical formation and lead to a substantial recovery of the major antioxidant enzymes, thus limiting oxidative damage to the liver[10-12,17]. It is shown that melatonin administration reduces histopathological signs of liver injury such as focal necrosis and apoptosis[17,27]. There are a few studies about the protective effect of low dose of melatonin against oxidative stress[27,37]. In the present study, histopathological evidence of parenchymatous injury was significantly reduced in the BDL+melatonin group (P<0.05). Portal fibrosis was not evident in this group. Additionally, melatonin administration prevented the GSH decrease and reduced significantly lipid peroxidation products. Montilla et al[10], reported significant differences in tissue and plasma MDA and GSH levels between non-treated and low dose of melatonin-treated rats. Lower levels of hepatic NOX in the BDL+melatonin group than in the BDL group (P<0.05) may support the role of melatonin on reducing free radical generation.
As a conclusion, the present study confirms the association between hepatic injury and increased oxidative stress, possibly mediated by NO. We have proved that even low dose of melatonin is efficient reducing the negative parameters of cholestasis, the degree of oxidative stress, and provided a significant hepatoprotective effect against liver injury secondary to ligation of biliary duct. Finally, we suggest that further studies comparing higher doses of melatonin should be performed in order to determine if the effect of melatonin is dose-dependent.