Published online Apr 7, 2005. doi: 10.3748/wjg.v11.i13.1917
Revised: July 29, 2004
Accepted: August 25, 2004
Published online: April 7, 2005
AIM: To evaluate the influence of avidin chase on the side effects of radioimmunotherapy (RIT) in nude mice bearing human colon carcinoma and therapeutic outcome.
METHODS: Purified anti-CEA monoclonal antibody (McAb) was biotinylated with NHS-biotin, and then radiolabeled with 188Re by the direct method. 188Re-labeled biotinylated anti-CEA McAb (188Re-CEA McAb-Bt) was intravenously injected followed by intravenous injection of avidin after 24 h. SPECT imaging and biodistribution study were performed at 28-48 h after the injection of 188Re-CEA McAb-Bt. Three groups of nude mice subcutaneously grafted with human colon carcinoma were treated 7 d after the graft. Mice in the avidin chase group received intravenous injection of 188Re-CEA McAb-Bt (11.1 MBq/20 μg) followed by intravenous injection of cold avidin (80 μg) after 24 h. Mice in the control group (treated group without avidin chase) only received the injection of 188Re-CEA McAb-Bt (11.1 MBq/20 μg), another control group (non-treated group) only received 0.1 mL normal saline solution. Toxicity was evaluated on the basis of change of body weight and peripheral WBC counts, and therapy effects were determined by variation in tumor volume. Histological analysis of tumors was also performed.
RESULTS: Avidin chase markedly accelerated the clearance of 188Re-CEA McAb-Bt from the blood and normal tissues. The tumor uptakes of 188Re-CEA McAb-Bt at 28 h were 5.90 and 6.42% ID/g, respectively, in chase group and in non-chase group, while the tumor-to-background (T/NT) ratios were 3.19 and 0.56, respectively. The tumor uptake was slightly decreased by avidin chase, but the T/NT ratios were increased. In treated groups the growth rate of body weight and the number of WBC decreased after injection of 188Re-CEA McAb-Bt, and the WBC counts recovered earlier in the group with avidin chase than in the group without avidin chase. Compared to the non-treated group, treated groups with and without avidin chase showed significant anti-tumor effects.
CONCLUSION: Avidin chase can effectively reduce the side effects of RIT, and improve therapeutic efficacy.
- Citation: Li GP, Wang YX, Huang K, Zhang H, Zhang CF. Avidin chase reduces side effects of radioimmunotherapy in nude mice bearing human colon carcinoma. World J Gastroenterol 2005; 11(13): 1917-1921
- URL: https://www.wjgnet.com/1007-9327/full/v11/i13/1917.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i13.1917
A relatively low tumor-to-background ratio is the major problem in radioimmunoimaging (RII), and high background activity is the limiting factor in radioimmunotherapy (RIT). This is due to the high levels of circulating radioactive antibodies and in turn, their slow pharmacokinetics. Enhancing the clearance of unbound circulating radiolabeled antibody and increasing the tumor-to-non-tumor (T/NT) ratio are important for RIT. Various strategies have been proposed to overcome the background problem, including computed background subtraction, use of second antibody, and antibody fragments, etc.[1]. Recently the extraordinary high affinity of avidin (Av) and streptavidin (SA) to biotin (Bt) has been utilized in pretargeting technique for delivering radiolabeled biotin derivatives suitable for imaging and therapy to target-bound streptavidin- or avidin-conjugated monoclonal antibodies (McAb)[2,3]. Preliminary reports using this technique are available in both experimental and clinical trials, and great success has been achieved[4-6]. Pretargeting technique based on the avidin-biotin system for tumor RII and RIT provides a new approach to reduce background activity in order to enhance T/NT ratio in the study of tumor targeting[7-9]. A new immunoimaging method using a radiolabeled biotinylated McAb and subsequent injection of avidin as a chaser can achieve a better antibody biodistribution for both immunoimaging and RIT when compared with conventional methods[10,11]. In the present study, 188Re was labeled to biotinylated anti-CEA McAb with a direct labeling method, and the avidin chase method was applied to RIT to reduce the hematologic side effects. The effects of avidin chase on biodistribution, side effects, and outcome of RIT were evaluated in nude mice bearing human colon carcinoma.
N-hydroxysuccinimidobiotin (BNSH), avidin, sodium gluconate, 2-mercaptoethanol (2-ME) and stannous chloride (SnCl2) were purchased from Sigma Chemical Co. (St. Louis, MO). 188ReO4- was supplied by the China Institute of Atomic Energy. All the chemical reagents used in this study were analytically pure, and prepared with deionized water.
CEA McAb was obtained from Shanghai Institute of Immunology. BNSH/dimethyl sulfoxide (DMSO) solution was added into the bicarbonate buffer solution containing the antibody at a ratio of 20:1. The mixture was gently stirred at room temperature for 1 h and finally purified with a Sephadex G50 chromatography. Indirect enzyme-linked immunosorbent assay (ELISA) was performed to assess the activity of biotin, and the purity of biotinylated antibody was determined by SDS-PAGE.
A direct labeling method was used in this study based on the method previously described by Griffiths et al[12], with some modifications. First biotinylated McAb was reduced by a 5000-fold molar excess of 2-ME for 30 min in order to reduce disulfide bonds of the antibody to mercapto groups. Then the reduced antibodies were purified by Sephadex G-50. The purified reduced antibodies were added to 0.2-1.0 mL of solution of 188ReO4- (185-370 MBq) and stannous chloride (SnCl2) solution dissolved in sodium gluconate solution. And the mixtures were incubated at room temperature for 2 h.
The radiochemical yield and the labeling efficiency of 188Re-CEA McAb-Bt were determined by paper chromatography using two developing systems. System 1 used No.1 Xinhua filter paper as solid phase and 50 mmoL/L acetic acid as eluent, Rf (188Re-CEA McAb-Bt) = 0.0, Rf (188ReO4-) = 0.7, Rf (Re colloid) = 0.0. System 2 used No.1 Xinhua filter paper impregnated with 5 g/L human serum albumin as solid phase and the mixture of ethanol, ammonium hydroxide, water (2:1:5) as eluent, Rf (188Re-CEA McAb-Bt) = 0.9, Rf (188ReO4-) = 0.7, Rf (Re colloid) = 0.0. The immunoreactivity of the labeled McAb was tested in indirect ELISA.
Human colon carcinoma cell line was heterotransplanted by subcutaneous injection of 1×107 LoVo cells in the thigh. The tumors growing to approximately 1 cm in diameter were cut into tiny pieces and suspended in normal saline, aspirated and injected (approximately 0.2 mL) subcutaneously into the forelimb of BALB/C nude mice (4-5 wk old). After the tumor grew to a volume of 0.5-1.0 cm3, the mice were used for subsequent study of biodistribution and RIT.
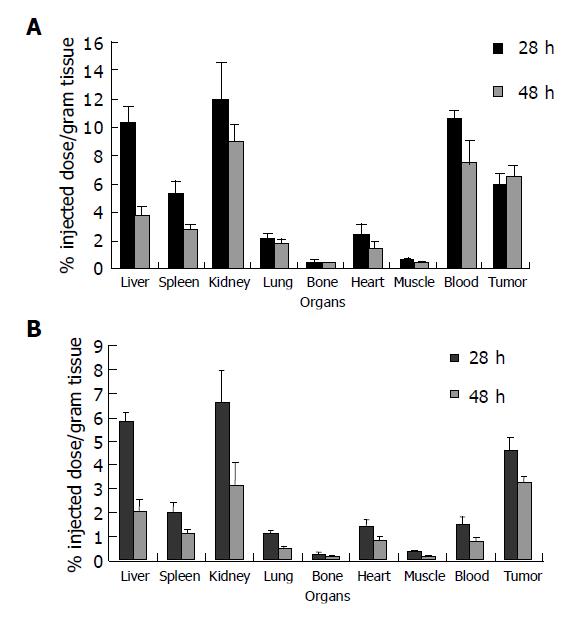
Twelve nude mice bearing human colon carcinoma were randomly divided into two groups, six in each group. Mice received an intravenous injection of 188Re-CEA McAb-Bt (3.7 MBq/20 μg), and mice of the avidin chase group received an intravenous injection of 80 μg avidin at 24 h. γ-imaging was performed on the mice of each group at 28 and 48 h, respectively, after injection of 188Re-CEA McAb-Bt. The mice were killed at each time point following the imaging, blood and various organs were removed and tumoral and normal tissue samples were washed in heparinized PBS and weighed, and their radioactivity counts were determined. The percent of injected dose per gram of tissue (%ID/g) and tumor-to-non-tumor (T/NT) ratio were calculated.
Grouping of the mice Fifteen tumor-bearing mice were randomly divided into three groups (five each group): treated group with avidin chase, treated group without avidin chase, and non-treated group. The treated mice without chase received a single intravenous injection of 188Re-CEA McAb-Bt (11.1 MBq/20 μg). The treated mice with chase received an intravenous injection of avidin (80 μg) at 24 h after injection of radiolabeled McAb (11.1 MBq/20 μg). The non-treated mice received an injection of normal saline (100 μL) only.
Observation of inhibiting effect on tumor growth During treatment tumor size was monitored by weekly measurement of the length (a) and width (b) of the tumor using a caliper for 5 wk. Estimated tumor volume (V) was calculated according to the formula: V = 1/6πab2. The inhibition rate (IR) of tumor growth was calculated according to the following formula: IR = [(mean tumor volume of the non-treated group-mean tumor volume of the treated group)/(mean tumor volume of the non-treated group)]×100%.
Monitoring of side effects Toxicity was monitored once a week principally by loss of body weight and WBC counts. Pathological examination was also performed in these mice at the end of the treatment study. The mice were killed with their organs isolated, weighed, fixed in 40 g/L formaldehyde solution, embedded in paraffin, cut into 4 μm sections and then stained with hematoxylin and eosin (HE) for microscopic observation.
The data were expressed as mean±SD. Student’s t test was used to analyze the statistical differences in %ID/g and T/NT ratio, tumor size, body weight and WBC counts among the groups. Differences were considered statistically significant when P values were less than 0.05.
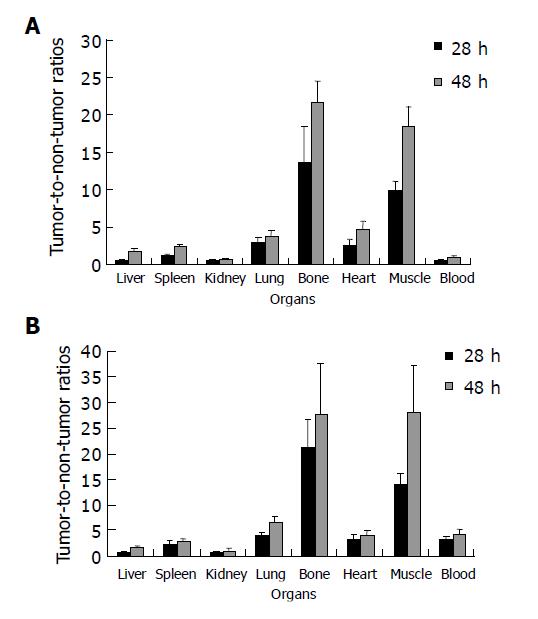
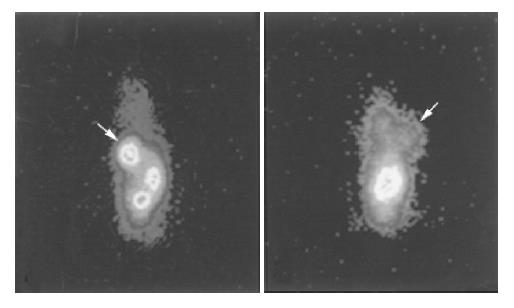
Biodistribution data in tumor-bearing nude mice are shown in Figure 1. The radioactivity levels in blood were 1.50±0.31 and 0.77±0.15 %ID/g, respectively, in the group with avidin chase 4 and 24 h after administration of avidin chase, and 10.47±0.63 and 7.35±1.60%ID/g, respectively, in the group without avidin chase. The other normal organs in the group with avidin chase also showed a significant decrease of radioactivity. However, the tumor uptake of 188Re-CEA McAb-Bt was slightly decreased by avidin chase, which was less than that in the normal organs and blood, resulting in increased T/NT ratios after administration of avidin chase. As shown in Figure 2, the tumor-to-blood ratios were 3.19 and 4.34, respectively, in the avidin chase group at 28 and 48 h after administration of 188Re-CEA McAb-Bt, and only 0.56 and 0.90, respectively, in the group without avidin chase. Tumor-to-normal tissue ratios in most organs were also higher in the group with avidin chase than in the group without avidin chase. The immunoimaging findings also supported the data obtained in the biodistribution studies. At 4 h after avidin chase injection, the xenografted tumor was clearly visualized in the avidin chase group, while in the non-chase group the xenografted tumor was not visualized at the same time point due to high blood background (Figure 3).
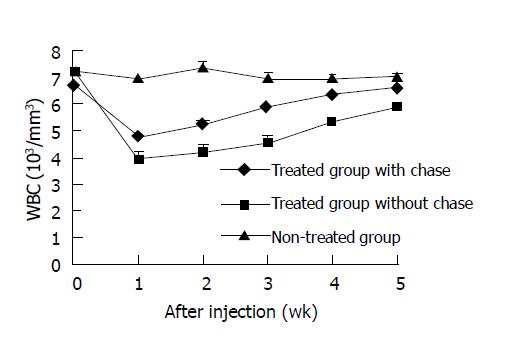
Hematologic toxicity was assessed by peripheral WBC counting (Figure 4). Compared with non-treated group, the number of peripheral blood WBC in treated groups decreased after injection of 11.1 MBq of 188Re-CEA McAb-Bt, reaching the nadir at wk 1. In the non-chase group, WBC number decreased by 45.3%, but only 29.5% in the chase group. The WBC counts recovered by wk 5 in the chase group, whereas the WBC recovery was slower in the non-chase group.
The body weight in non-treated group increased continuously during the observation period. The non-treated mice gained 20.0% of their initial body weight 5 wk after treatment. However, the increment of body weight in treated group was slow. The treated mice gained 10.6% and 12.2% of their initial body weight 5 wk after treatment in the non-chase and chase groups.
Microscopic observation revealed no obvious tissue radiation damage in such vital organs as the heart, liver, spleen, lungs and brain in all nude mice at the end of treatment.
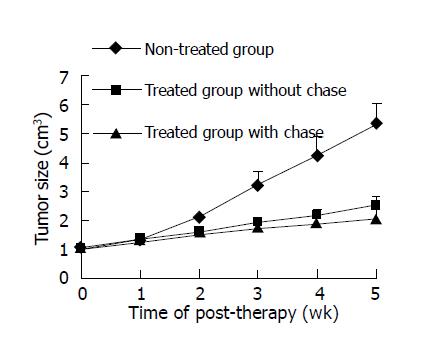
In the first week of treatment, the tumor volume showed little difference among the groups, while in the 2nd wk slower tumor growth in the treated group was observed. In the 5th wk, the tumor volume was obviously smaller in the treated group than that in the non-treated group (P<0.001) (Figure 5). If the tumor IR in the non-treated group was considered as zero, the tumor IR in the fifth week after treatment was 61.79% and 53.0% in the chase and non-chase groups, respectively.
Histopathological evidence indicated that in treated group radioactive damage in tumor tissues was shown as karyopyknosis, structure disorder and rupture of tumor cells, and in some regions the tumor cells were necrotic or disappeared. While in the non-treated group tumor tissue was characterized by the absence of necrosis, and took on typical form of carcinoma cells.
RIT is a therapeutic approach that uses antibodies directed against tumor-associated antigens to carry cytotoxic radionuclides to antigen-expressing tumor tissue. The success of RIT depends on high tumor uptake with high tumor-to normal tissue ratios and a homogeneous accumulation of radiolabels in the tumors. However, several problems still prevent its full clinical exploitation[13,14]. Radioimmunotargeting is limited by the persistence of radiolabeled antibody in circulation, which results in low tumor to blood ratios. This slow antibody clearance from blood and non-target tissues delays the time at which imaging can be carried out, and also therapy is limited by the potential damage to normal tissues. To alleviate this problem various clearing approaches have been used to reduce blood background levels. The first method involves the use of a second antibody reactive with the first anti-tumor antibody administered as a clearing agent, in which efficient clearing of the immune complexes formed in blood is achieved via the reticuloendothelial system[15,16]. Another method involves the use of biotinylated anti-tumor antibodies with avidin administered as a clearing agent. This system uses the very high affinity of avidin for biotin and also the fast clearance of avidin-complexed biotinylated antibodies[11]. The ability of avidin to clear biotinylated antibodies from the circulation was first reported by Sinitsyn et al[11]. A preliminary study for radioimmunoguided surgery by Paganelli et al[8] showed that avidin administration after radiolabeled biotinylated antibody injection results in successful blood clearance in a patient. Avidin has also been used successfully by Norrgren et al[17], to reduce circulating 125I-biotinylated anti-tumor antibody via extracorporeal immunoadsorption on an agarose-avidin column, resulting in improved tumor to non-tumor ratios.
Kobayashi et al[10], have achieved tumor-targeting with low background radioactivity by using an avidin chase method, in which a radiolabeled biotinylated McAb is injected first, and after the optimal time for McAb to accumulate in the tumor, avidin is administered. Since avidin quickly forms complexes with the unbound radiolabeled biotinylated McAb in circulation, the complexes are rapidly taken up by liver and spleen, thus resulting in rapid clearance of radioactivity from the blood and normal organs. In the present study, we injected 188Re-CEA McAb-Bt followed by avidin injection as a chase to decrease the background radioactivity and speed up the excretion of radionuclides without decreasing the dose of radioactivity delivered to the target tumor. In order to obtain sufficiently high accumulation of radioactivity in the tumor, avidin as clearing agent was injected at 24 h after the injection of 188Re-CEA McAb-Bt. The dose of avidin used in this study was four times that of injected McAb on a weight basis, which was supposed to be sufficient to form a complex with biotinylated McAb in circulation. Avidin chase rapidly cleared the circulating radiolabeled McAb and efficiently decreased the blood background levels. Tumor-to-blood ratio was significantly higher at 4 h after injection of avidin chase in the chase group than in the non-chase group, and the tumor was also clearly visualized in the chase group, suggesting that avidin chase elevates the T/NT ratios and improves the quality of imaging.
Compared with the non-chase group, avidin chase produced a significant tumor-inhibition effect but caused little radiation-related damage to normal tissues or organs. The bone marrow toxicity and body weight loss were milder in the avidin chase group than in the non-chase group. The WBC number was decreased by 45.3% in the group without chase, and only by 29.5% in the chase group. These results are similar to those of previous report, in which a second antibody was used as a clearing agent[18]. Sharkey et al[19], also reported that tumor uptake is reduced when second antibody is used as a clearing agent. However this reduction in tumor uptake induced by avidin chase is milder than that by the second antibody method. Meanwhile, this decrease in tumor radioactivity is less than that in normal organs and blood, resulting in increased T/NT ratios after avidin chase injection.
A single intravenous injection of 188Re-CEA McAb-Bt at the dose of 11.1 MBq produced significant anti-tumor efficacy in nude mice bearing human colon carcinoma. Since initial uptake of radiolabeled McAb in tumor xenografts was higher in both chase and non-chase groups, irradiation with a higher dose rate at earlier times may be more cytotoxic and effective than a lower dose rate irradiation at later times[20]. Bone marrow toxicity and body weight loss could not be avoided due to the high radioactivity in circulation at earlier times. However, the significantly decreased blood radioactivity after avidin chase injection may facilitate earlier recovery from these side effects.
Rhenium-188 is thought to be an ideal radionuclide for RIT due to its physical half-life of 16.9 h and β emission of 2.12 MeV. And pretargeting technique based on the avidin-biotin system for tumor RII and RIT provides new possibility to reduce background activity in order to enhance the T/NT ratio in the study of tumor targeting. In view of the above considerations, we selected radionuclide rhenium-188 to label biotinylated anti-CEA McAb for RIT of colon carcinoma, and intravenously injected avidin chase as a clearing agent to remove the free, tumor-unbound McAb from the circulation. The results showed that avidin-induced blood clearance of biotinylated McAb was produced and favorable results had been achieved in the therapy of colon carcinoma in animal models. These results provide much useful information for further investigation and clinical applicability of avidin chase protocols in RII and RIT.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Bian HJ, Chen ZN, Deng JL. Direct technetium-99m labeling of anti-hepatoma monoclonal antibody fragment: a radioimmunoconjugate for hepatocellular carcinoma imaging. World J Gastroenterol. 2000;6:348-352. [PubMed] |
| 2. | Alvarez-Diez TM, Polihronis J, Reilly RM. Pretargeted tumour imaging with streptavidin immunoconjugates of monoclonal antibody CC49 and 111In-DTPA-biocytin. Nucl Med Biol. 1996;23:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Fritzberg AR. Antibody pretargeted radiotherapy: a new approach and a second chance. J Nucl Med. 1998;39:20N-22N, 36N. [PubMed] |
| 4. | Hnatowich DJ, Virzi F, Rusckowski M. Investigations of avidin and biotin for imaging applications. J Nucl Med. 1987;28:1294-1302. [PubMed] |
| 5. | Kalofonos HP, Rusckowski M, Siebecker DA, Sivolapenko GB, Snook D, Lavender JP, Epenetos AA, Hnatowich DJ. Imaging of tumor in patients with indium-111-labeled biotin and streptavidin-conjugated antibodies: preliminary communication. J Nucl Med. 1990;31:1791-1796. [PubMed] |
| 6. | Paganelli G, Belloni C, Magnani P, Zito F, Pasini A, Sassi I, Meroni M, Mariani M, Vignali M, Siccardi AG. Two-step tumour targetting in ovarian cancer patients using biotinylated monoclonal antibodies and radioactive streptavidin. Eur J Nucl Med. 1992;19:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Paganelli G, Pervez S, Siccardi AG, Rowlinson G, Deleide G, Chiolerio F, Malcovati M, Scassellati GA, Epenetos AA. Intraperitoneal radio-localization of tumors pre-targeted by biotinylated monoclonal antibodies. Int J Cancer. 1990;45:1184-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Paganelli G, Stella M, Zito F, Magnani P, De Nardi P, Mangili F, Baratti D, Veglia F, Di Carlo V, Siccardi AG. Radioimmunoguided surgery using iodine-125-labeled biotinylated monoclonal antibodies and cold avidin. J Nucl Med. 1994;35:1970-1975. [PubMed] |
| 9. | Stella M, De Nardi P, Paganelli G, Magnani P, Mangili F, Sassi I, Baratti D, Gini P, Zito F, Cristallo M. Avidin-biotin system in radioimmunoguided surgery for colorectal cancer. Advantages and limits. Dis Colon Rectum. 1994;37:335-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Kobayashi H, Sakahara H, Hosono M, Yao ZS, Toyama S, Endo K, Konishi J. Improved clearance of radiolabeled biotinylated monoclonal antibody following the infusion of avidin as a "chase" without decreased accumulation in the target tumor. J Nucl Med. 1994;35:1677-1684. [PubMed] |
| 11. | Sinitsyn VV, Mamontova AG, Checkneva YY, Shnyra AA, Domogatsky SP. Rapid blood clearance of biotinylated IgG after infusion of avidin. J Nucl Med. 1989;30:66-69. [PubMed] |
| 12. | Griffiths GL, Goldenberg DM, Knapp FF, Callahan AP, Chang CH, Hansen HJ. Direct radiolabeling of monoclonal antibodies with generator-produced rhenium-188 for radioimmunotherapy: labeling and animal biodistribution studies. Cancer Res. 1991;51:4594-4602. [PubMed] |
| 13. | Blumenthal RD, Lew W, Reising A, Soyne D, Osorio L, Ying Z, Goldenberg DM. Anti-oxidant vitamins reduce normal tissue toxicity induced by radio-immunotherapy. Int J Cancer. 2000;86:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Vriesendorp HM, Quadri SM, Andersson BS, Dicke KA. Hematologic side effects of radiolabeled immunoglobulin therapy. Exp Hematol. 1996;24:1183-1190. [PubMed] |
| 15. | Pedley RB, Dale R, Boden JA, Begent RH, Keep PA, Green AJ. The effect of second antibody clearance on the distribution and dosimetry of radiolabelled anti-CEA antibody in a human colonic tumor xenograft model. Int J Cancer. 1989;43:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Goldenberg DM, Sharkey RM, Ford E. Anti-antibody enhancement of iodine-131 anti-CEA radioimmunodetection in experimental and clinical studies. J Nucl Med. 1987;28:1604-1610. [PubMed] |
| 17. | Norrgren K, Strand SE, Nilsson R, Lindgren L, Sjögren HO. A general, extracorporeal immunoadsorption method to increase the tumor-to-normal tissue ratio in radioimmunoimaging and radioimmunotherapy. J Nucl Med. 1993;34:448-454. [PubMed] |
| 18. | Blumenthal RD, Sharkey RM, Snyder D, Goldenberg DM. Reduction by anti-antibody administration of the radiotoxicity associated with 131I-labeled antibody to carcinoembryonic antigen in cancer radioimmunotherapy. J Natl Cancer Inst. 1989;81:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Sharkey RM, Primus FJ, Goldenberg DM. Second antibody clearance of radiolabeled antibody in cancer radioimmunodetection. Proc Natl Acad Sci USA. 1984;81:2843-2846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Muthuswamy MS, Roberson PL, Buchsbaum DJ. A mouse bone marrow dosimetry model. J Nucl Med. 1998;39:1243-1247. [PubMed] |