Published online Mar 28, 2005. doi: 10.3748/wjg.v11.i12.1822
Revised: May 27, 2004
Accepted: June 17, 2004
Published online: March 28, 2005
AIM: To study the stereoselectivity of phase II glucuronidation metabolism of side-chain propranolol in Chinese Han population.
METHODS: Sixteen adult Chinese Han volunteers with an average age of 20 years were given a single oral dose of 20 mg racemic propranolol. Human urine at indicated time after administration was collected and S-(-)-propranolol glucuronide and R-(+)-propranolol glucuronide were determined simultaneously by using RP-HPLC.
RESULTS: The mean values of k were 0.19±0.04 h-1 and 0.28±0.06 h-1, of t1/2 3.56±0.73 h and 2.45±0.50 h, of Tmax 2.21±0.45 and 1.75±0.33 h, and of Xu0-24 5.65±0.98 and 2.95±0.62 μmoL for S-(-)- and R-(+)-propranolol glucuronide, respectively. The cumulative excretion percentages in urine of doses were 14.7±2.46% and 7.68±1.60% for S-(-)- and R-(+)-propranolol glucuronide, respectively. The results showed the elimination rate constant k of S-(-)-propranolol glucuronide was less than that of R-(+)-propranolol glucuronide; and the elimination half-life (t1/2), Tmax and the cumulative excretion amount(Xu0-24) of R-(+)-propranolol glucuronide were significantly less than that of S-(-)-propranolol glucuronide.
CONCLUSION: The propranolol glucuronidation of the side-chain undergoes stereoselective excretion in Chinese Han population after an oral administration of racemic propranolol.
- Citation: Luan LJ, Shao Q, Ma JY, Zeng S. Stereoselective urinary excretion of S-(-)- and R-(+)-propranolol glucuronide following oral administration of RS-propranolol in Chinese Han subjects. World J Gastroenterol 2005; 11(12): 1822-1824
- URL: https://www.wjgnet.com/1007-9327/full/v11/i12/1822.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i12.1822
Propranolol [1-isopropylamino-3-(1-naphthyloxy)-2-propanol], available commercially as the racemic mixture, is a nonselective β-adrenergic blocking agent used in the treatment of hypertension, angina pectoris and cardiac arrhythmias. Since S-(-)-propranolol is about 100 times more potent a β-blocker than its optical antipode, significant differences in their disposition may be important clinically[1-3]. The primary metabolic pathways of propranolol are glucuronidation, side-chain oxidation and ring oxidation. The metabolic products arise from naphthalene-ring hydroxylation, N-dealkylation of the isopropanolamine side-chain and side-chain O-glucuronidation[4,6,7]. Glucuronidation represents a major pathway which enhances the elimination of many lipophilic xenobiotics and endobiotics to more polar compounds and, thus, more readily excreted in bile or urine[8]. It has been reported that stereoselective glucuronidation metabolism of propranolol occurred in whites and blacks[9-11], but so far, there has been no such information from Chinese Han subjects. The aim of the present study was to provide further information of the stereoselective side-chain glucuronidation in the urinary excretion of the propranolol enantiomers glucuronides conjugate after oral dosing with racemic propranolol in Chinese Han volunteers.
Racemic propranolol, R-(+) and S-(-)-propranolol were supplied by Sigma Chemical Co. (St. Louis, MO, USA). Propranolol glucuronide was biosynthesized according to Yu’s methods[12]. All other chemicals and solvents were of an analytical or chromatographic grade and obtained commercially.
Chromatographic determination of S-(-)-propranolol glucuronide and R-(+)-propranolol glucuronide was performed by using a Shimadzu HPLC system equipped with LC-10AT VP pumps coupled to a manual injector with a 20 μL fixed loop, a 5-μm reverse phase column (C18, 250 mm×4.6 mm i.d ), and an SPD10A VP Fluorescence detector. Column temperature was set at 30 °C. The chrom-atographic data were collected and processed on an Epper chromatopac station version (Zhejiang University, Hangzhou, China). The mobile phase consisted of 67 mmol/L KH2PO4 buffer-methanol (60:40, v/v, pH 3.5) with a flow rate of 1.0 mL/min. The chromatographic peaks of eluted components were monitored at Ex310 nm and Em339 nm. The concentrations of S-(-)-propranolol glucuronide and R-(+)-propranolol glucuronide were calculated by integration of their chromatographic peaks.
This study was approved by the Ethics Committee of College of Pharmaceutical Sciences, Zhejiang University. Sixteen adult Han volunteers with an average age of 20 years (18-22 years) and an average weight of 55 kg (52-58 kg) participated in this study. The volunteers were judged to be in good health on the basis of their medical history, physical examination and laboratory profiles, which were performed within two weeks before the study. The volunteers were given 150 mL of water after excreting urine. Then they were given a single oral dose of 20 mg racemic propranolol tablet with 150 mL of water on an empty stomach. During the investigation period, intake of other drugs and of alcohol was not allowed. Urine samples were collected just before dosing and at 1, 2, 4, 6, 8, 10, 12 and 24 h after dosing. The volumes of the urine samples were measured after collection and the urine samples were stored at -20 °C until analysis.
The urine samples were allowed to stand and warmed to room temperature, then filtered. The filtrates, diluted with water if necessary, were spun by centrifugation for 10 min at 3500 r/min. Twenty microliters of the urine supernatants was injected into the HPLC system and then assayed.
The apparent terminal elimination rate constant, k, was calculated through least-squares regression analysis of urine excretion rate-mid-point time date over the terminal log-linear disposition phase. The elimination half-life (t1/2) was calculated as 0.693/k. The accumulative excretion from zero to 24 h in urine (Xu0→24) was estimated as 0-24Cu×△T, with Cu representing the concentration of drug at the collection interval (△T). The excretion percentage in urine was calculated by dividing oral dosage by Xu0→24. Student’s t-test was used to evaluate the statistical significance of differences.
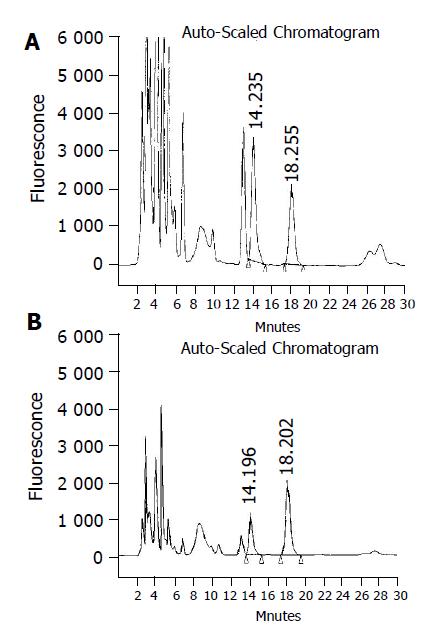
The drug free human urine spiked with S-(-)- and R-(+)-propranolol glucuronides and true samples was analyzed simultaneously to identify the chromatographic peaks of glucuronidation metabolites. The S-(-)- and R-(+)-propranolol glucuronide in urine was confirmed by hydrolyzing with β-D-glucuronidase. There was no peak found at the same retention time of S-(-)- and R-(+)-propranolol glucuronide in the chromatograms of blank human urine. The R-(+)-propranolol glucuronide was eluted at about 14.2 min and S-(-)-propranolol glucuronide about 18.2 min, propranolol about 27.5 min and the resolution between two enantiomers was more than 2.5 (Figure 1).
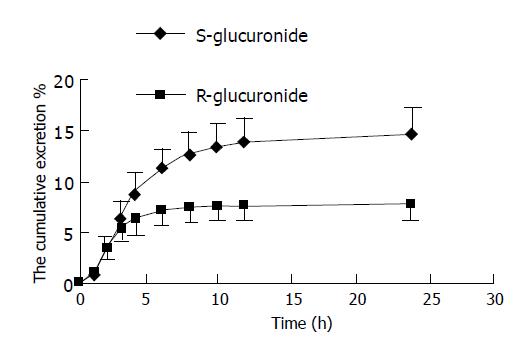
The excretion amounts of S-(-)- and R-(+)-propranolol glucuronide in urine at indicated time after an oral administration of 20 mg RS-(±)-propranolol tablet are shown in Table 1 and the mean values of k, t1/2, Tmax and [Xu]0→24 of S-(-)- and R-(+)-propranolol glucuronide for 16 healthy Chinese Han volunteers are presented in Table 2. The cumulative excretion percentage of S-(-)- and R-(+)-propranolol glucuronide of dose in human urine after an oral administration of 20 mg racemic propranolol tablet is shown in Figure 2.
The stereoselectivity of propranolol glucuronidation has been found in whites and blacks at different doses and by using different analytical methods. Pham-Huy et al, described an analytical method to determine the S- and R-propranolol conjugates indirectly. The glucuronide conjugates are cleaved prior to extraction by incubating and then the enantiomers are derivatized with R(+)-phenylethylisocyanate as chiral derivatization reagent. The results indicated that S-propranolol conjugates showed higher concentrations than R-propranolol conjugates in plasma and urine[9]. Silber et al[10], reported plasma concentrations of S-(-)-propranolol glucuronide conjugate were greater than that for R(+)-propranolol conjugate and the terminal elimination half-lives of S-(-)-propranolol glucuronide conjugate were longer than for the R(+)-enantiomer at all doses in four healthy adults. Racial differences in propranolol enantiomer kinetics following simultaneous intravenous and oral administration in 12 white and 13 black healthy males have been reported and there were trends (P>0.05<0.10) toward higher R-propranolol glucuronidation in blacks compared with whites[11]. There are some merits for the disposition study using urine-drug excretion data, such as more information of metabolism could be obtained, urine samples were easily acquired from the volunteers; the larger sample volume could be used and the assay sensitivity could be improved; and the pretreatment of urine sample was simpler for assay in comparison with that of blood, etc.
It has been reported that Chinese are more sensitive to the β-blocking and hypotensive effects of propranolol than Caucasians[5]. Our study is the first on the feature of stereoselective glucuronidation metabolism of propranolol in urine of Chinese Han subjects. The S- and R-propranolol glucuronides were directly separated and simultaneously assayed by using RP-HPLC without hydrolysis and chiral derivatization. The results from Table 1, Figures 1 and 2 showed that in the first hour, there were trends toward higher cumulative excretion of R-propranolol glucuronide in urines without significant difference. The ratio of S-/R-propranolol glucuronide was reversed 2 h after oral administration from <1 to >1 and up to 1.86 at 24 h (Figure 2). The experimental results indicated that the elimination rate constant k of S-(-)-propranolol glucuronide was less than that of R-(+)-propranolol glucuronide; and the elimination half-life (t1/2), Tmax and the cumulative excretion amount (Xu0-24) of R-(+)-propranolol glucuronide were significantly less than that of S-(-)-propranolol glucuronide (Table 2). The excretion percentage of S-(+)- and R-(+)-propranolol glucuronide was 14.7% and 7.68% of the dose, respectively. The total amounts excreted in urine were 22.4% of the amounts ingested, which is higher than that in white people with 17%.
In conclusion, the results obtained suggest that the urinary excretion of glucuronidation of the side-chain was stereoselective in Chinese Han populations after an oral administration of racemic propranolol.
| 1. | Silber B, Riegelman S. Stereospecific assay for (-)- and (+)-propranolol in human and dog plasma. J Pharmacol Exp Ther. 1980;215:643-648. [PubMed] |
| 2. | Li X, Zeng S. Stereoselective propranolol metabolism in two drug induced rat hepatic microsomes. World J Gastroenterol. 2000;6:74-78. [PubMed] |
| 3. | Masubuchi Y, Hosokawa S, Horie T, Suzuki T, Ohmori S, Kitada M, Narimatsu S. Cytochrome P450 isozymes involved in propranolol metabolism in human liver microsomes. The role of CYP2D6 as ring-hydroxylase and CYP1A2 as N-desisopropylase. Drug Metab Dispos. 1994;22:909-915. [PubMed] |
| 4. | Luan LJ, Shao Q, Zhang XH, Zeng S. Effects of microsome enzyme induced by phenobarbarbital on the stereoselectivity of recemic propranolol glucuronidation metabolism. Zhejiang DaXue XueBao YiXueBan. 2004;33:7-10. [PubMed] |
| 5. | Zhou HH, Shay SD, Wood AJ. Contribution of differences in plasma binding of propranolol to ethnic differences in sensitivity. Comparison between Chinese and Caucasians. Chin Med J (Engl). 1993;106:898-902. [PubMed] |
| 6. | Zhou Q, Yao TW, Zeng S. Chiral reversed phase high-performance liquid chromatography for determining propranolol enantiomers in transgenic Chinese hamster CHL cell lines expressing human cytochrome P450. J Biochem Biophys Methods. 2002;54:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Johnson JA, Herring VL, Wolfe MS, Relling MV. CYP1A2 and CYP2D6 4-hydroxylate propranolol and both reactions exhibit racial differences. J Pharmacol Exp Ther. 2000;294:1099-1105. [PubMed] |
| 8. | Tateishi T, Fujimura A, Shiga T, Ohashi K, Ebihara A. Influence of aging on the oxidative and conjugative metabolism of propranolol. Int J Clin Pharmacol Res. 1995;15:95-101. [PubMed] |
| 9. | Pham-Huy C, Sahui-Gnassi A, Saada V, Gramond JP, Galons H, Ellouk-Achard S, Levresse V, Fompeydie D, Claude JR. Microassay of propranolol enantiomers and conjugates in human plasma and urine by high-performance liquid chromatography after chiral derivatization for pharmacokinetic study. J Pharm Biomed Anal. 1994;12:1189-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Silber B, Holford NH, Riegelman S. Stereoselective disposition and glucuronidation of propranolol in humans. J Pharm Sci. 1982;71:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Sowinski KM, Lima JJ, Burlew BS, Massie JD, Johnson JA. Racial differences in propranolol enantiomer kinetics following simultaneous i.v. and oral administration. Br J Clin Pharmacol. 1996;42:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Yu L, Luan L, Shao Q, Zeng S. Direct determination of S-(-)- and R-(+)-propranolol glucuronide in rat hepatic microsomes by RP-HPLC. Biomed Chromatogr. 2004;18:833-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |