Published online Mar 28, 2005. doi: 10.3748/wjg.v11.i12.1802
Revised: May 28, 2004
Accepted: June 25, 2004
Published online: March 28, 2005
AIM: To investigate the correlation among the presence and degree of gastric metaplasia of duodenal regenerating mucosa, the deformity of bulb and the recurrence of duodenal ulcer.
METHODS: A total of 99 patients with duodenal ulcer were treated with H2-antagonist with or without antimicrobial therapy. All patients received follow-up endoscopic examinations 6 wk after treatment. When the ulcer(s) were noted to be healed, two biopsies were taken from the ulcer scar for histological study of gastric metaplasia, and 4 biopsies were taken from antrum for Helicobacter pylori (H pylori) study. Out of these cases, 44 received further follow-up endoscopic examinations after 3, 6 and 12 mo respectively for studying the recurrence rate of duodenal ulcers. The correlation among ulcer recurrence, degree of gastric metaplasia of regenerating mucosa, bulbar deformity, and colonization of H pylori in the stomach was then studied.
RESULTS: The results showed that there was a strong correlation between the deformity of duodenal bulb and the degree of gastric metaplasia of regenerating duodenal mucosa. The recurrence rate of duodenal ulcer had a significant difference between patients with and without H pylori colonization in the stomach (P<0.001). The greater the degree of gastric metaplasia of duodenal regenerating mucosa, the higher the recurrence rate of duodenal ulcer (P = 0.021). The more deformed the duodenal bulb, the higher the incidence of recurrence of duodenal ulcer (P = 0.03).
CONCLUSION: There is a correlation among deformity of duodenal bulb, gastric metaplasia of duodenal regenerating mucosa and recurrence of duodenal ulcer. A more severely deformed duodenal bulb is closely related to a greater extent of gastric metaplasia. Both factors contribute to the recurrence of duodenal ulcer.
- Citation: Chang CC, Pan S, Lien GS, Liao CH, Chen SH, Cheng YS. Deformity of duodenal bulb, gastric metaplasia of duodenal regenerating mucosa and recurrence of duodenal ulcer: A correlated study. World J Gastroenterol 2005; 11(12): 1802-1805
- URL: https://www.wjgnet.com/1007-9327/full/v11/i12/1802.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i12.1802
Duodenal ulcer is the most common kind of peptic ulcer diseases in Taiwan. Healing of duodenal ulcer is not so difficult. However, the frequent recurrence of duodenal ulcer is still a big problem to physicians. The recurrence rate of duodenal ulcer was reported from 50% to 80% within one year[1-3]. Although Helicobacter pylori (H pylori) colonization has been considered as the most important factor related to the recurrence of duodenal ulcer, and eradication of H pylori has been proved to greatly reduce the recurrence rate[4-10]. Except for H pylori, there might be other factors contributing to the ulcer recurrence. Our previous studies revealed that histological maturity of healed duodenal ulcers and deformity of duodenal bulb might play a role in the recurrence of duodenal ulcer[11-15]. A 3- to 8 -year follow-up study (n = 448) revealed that about 20% of the patients with duodenal ulcer never experienced recurrence of duodenal ulcer after successful treatment, none of these “cured” patients had a moderate or severe degree of deformity of bulb. In contrast, patients with a marked deformity of bulb suffered from repeat recurrence of duodenal ulcer and even underwent surgical intervention[15]. It has been suggested that colonization of H pylori on the gastric type epithelium of the duodenum[16,17] could cause an active chronic duodenitis, leading to duodenal ulceration. Gastric metaplasia of the duodenal mucosa has been considered to be closely related to the development of duodenal ulcer. Based on the above recognition, we considered that there might be some relationships between gastric metaplasia of duodenal mucosa, bulbar deformity and duodenal ulcer recurrence. Therefore, we designed this prospective study to investigate the correlation among the presence and degree of gastric metaplasia of duodenal regenerating mucosa, the deformity of bulb and the recurrence of duodenal ulcer.
This study was approved by the Human Subjects Committee at our institution, and informed consent was obtained from all subjects prior to participation.
Upper gastrointestinal endoscopy was performed using an Olympus GIF-XQ200 endoscope (Olympus Optical Co., Ltd, Tokyo, Japan). Based on the endoscopic morphological patterns of the duodenal bulb, we divided duodenal ulcers into 3 types. Type I had a normal-shaped bulb, type II had a ridge across the bulb with pseudo-diverticulum formation (mild degree of deformity), type III had multiple ridges occupying the bulb (marked deformity).
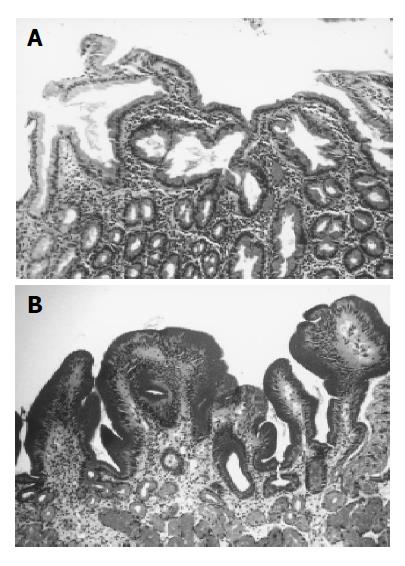
Histologically, we divided gastric metaplasia into 3 grades. Grade 0: no ectopic gastric mucosa to be noted (Figure 1A), Grade 1: less than one fourth of regenerating mucosa containing gastric foveolar epithelium, Grade 2: more than one fourth of regenerating mucosa containing gastric foveolar epithelium (Figure 1B). The correlation between ‘the type’ of duodenal ulcer and the presence and degree of gastric metaplasia was studied.
A total of 99 patients with endoscopically proven healed duodenal ulcer were enrolled in this study. Two biopsy specimens were obtained from the healed ulcer center during endoscopic examination. Four micrometer thick sections of tissue were prepared and underwent staining with HE and periodic acid Schiff (PAS) for light microscopic examinations to identify the presence and degree of gastric metaplasia. Another four biopsies were taken from the gastric antrum to examine H pylori infections using Campylobacter-like organism (CLO) test and histology with HE staining.
Ten normal healthy subjects were used as a control group for evaluating the incidence of gastric metaplasia in normal duodenal mucosae and the positive rate of H pylori infection in normal subjects.
All the 99 patients received anti-H pylori triple therapy for 2 wk and followed histamine 2-receptor antagonist treatment for 6-8 wk. The patients were not allowed to receive anti-ulcer drugs until attack of dyspepsia again. Follow-up endoscopic examinations were carried out at 3, 6, and 12 mo after cessation of medication in order to examine the condition of duodenal ulcers. The correlation among bulbar deformity, gastric metaplasia of regenerating duodenal mucosa, and the recurrence of duodenal ulcer was evaluated. The recurrence rate of duodenal ulcer in H pylori-infected and H pylori-eradicated patients was checked and the relationship between H pylori infection and gastric metaplasia or bulbar deformity was also studied.
Statistical analysis was conducted using Student’s t-test, χ2 analysis with Yates’ correction and Fisher’s exact test. The results were expressed as mean±SD.
Ninety (91%) out of the 99 patients were infected with H pylori before ulcer therapy. These 99 patients consisted of 71 males and 28 females, and they aged from 18 to 79 years with a mean age of 49.2±11.95 years.
Forty-four out of the 99 patients received further follow-up endoscopic examinations at 3, 6, and 12 mo after the ulcer(s) were endoscopically proved to be healed. Among these 44 patients, 14 had no deformity (Type I), 30 had deformity of bulb (16 Type II and 14 Type III). The mean age of the patients with types I, II, III ulcers was 46.6±14.56, 50.6±8.37, and 54.5±11.04 years, respectively. The differences in mean age types of duodenal ulcer were not statistically significant (P>0.01). Meanwhile, according to the regenerating duodenal mucosae of these 44 cases, 10 had grade 0, 10 had grade 1 and 24 had grade 2 gastric metaplasia. The mean age was 49.8±7.3, 49.5±13.2, and 53.0±13.3 years in patients with grade 0, grade 1, and grade 2 gastric metaplasia, respectively. It showed no significant difference between patients with different grades of gastric metaplasia (P>0.01).
As shown in Table 1, 6 patients (42.86%) with14 type I, 3 (18.75%) with 16 type II, and 1 patients (7.14%) with 14 type III ulcers had grade 0 gastric metaplasia. However, 57.14% of type I, 81.25% of type II, and 92.86% of type III ulcers were noted to have grade 1 or 2 gastric metaplasia. Type II or type III ulcers had a higher grade of gastric metaplasia than type I ulcer. The differences between type I and type II or III ulcers were statistically significant (P<0.04 in each).
| Bulb not deformed1 | Bulb deformed1 | Total | ||
| Type I | Type II | Type III | ||
| Grade 0 | 6 | 3 | 1 | 10 |
| Grade 1 | 4 | 4 | 2 | 10 |
| Grade 2 | 4 | 9 | 11 | 24 |
| Total | 14 | 16 | 14 | 44 |
Out of these 44 patients, 18 (40.9%) had H pylori colonization and 26 (59.1%) had no H pylori colonization at antrum of the stomach at the final endoscopy. In the H pylori-infected group, 15 patients (83.3%) were found to have ulcer recurrence within 12 mo after cessation of treatment. On the other hand, out of 26 H pylori-eradicated patients, 6 (23.1%) were found to have recurrence. The recurrence rate of duodenal ulcer was significantly different between these two groups (P<0.001). As to the deformity of bulb, 3 patients (21.43%) with 14 type I, 8 patients (50%) with 16 type II, and 10 patients (71.43%) with 14 type III ulcers were found to have recurrence, the differences in recurrence rate among different types were statistically significant (P = 0.03). As to the grade of gastric metaplasia of regenerating duodenal mucosa, 1 patients (10%) with 10 grade 0, 5 (50%) with 10 grade 1, and 15 patients (62.5%) with 24 grade 2 gastric metaplasia had ulcer recurrence within 12 mo. The differences in recurrence rate among the grades of gastric metaplasia of regenerating duodenal mucosa were statistically significant (P = 0.02).
Out of the 40 specimens obtained from duodenal mucosa of healthy subjects at the site of 0.5 to 1.0 cm distal from pyloric ring (either anterior or posterior wall of the bulb), 33 (82.5%) were grade 0 and 7 (17.5%) were grade 1 gastric metaplasia. The incidence was significantly less than that of healed duodenal ulcers (P<0.05). The positive rate of H pylori colonization was 60% in these group, it seemed lower than that of patients with duodenal ulcers. However, there was no statistically significant difference between these two groups (60% vs 91%, P>0.05).
It is well known that periodic acid Schiff (PAS) stain is the best method to identify gastric metaplasia of duodenal mucosa. However, gastric metaplasia is also easily identified by hematoxylin and eosin (HE) staining by the presence of clusters of epithelial cells with distinct apical cytoplasmic vacuoles and without a brush border. This gastric-type epithelium is easily distinguished from adjacent duodenal epithelial cells by examining cytoplasmic vacuoles and brush border[18]. In the present study, tissue preparations were treated both with HE and PAS staining to assess the presence and degree of gastric metaplasia of duodenal mucosa in patients with healed duodenal ulcer and in healthy subjects.
Although surface gastric metaplasia and duodenitis are closely associated, they have not been considered to share a common pathogenesis[19]. Furthermore, Fitzgibbons et al[18] postulated that the occurrence of gastric metaplasia was unrelated to the presence of severe duodenitis. Therefore, the present study did not investigate the correlation between gastric metaplasia and duodenitis, we chiefly studied the possible correlation between the presence and extent of gastric metaplasia of duodenal mucosa and the deformity of duodenal bulb in patients with healed duodenal ulcer.
It has been reported that gastric metaplasia of duodenal mucosa could be identified in 22-25% of asymptomatic volunteers[18,19] and in 77.6% to 100% of patients with active duodenal ulcer[19-21]. As to the patients with healed duodenal ulcer, Carrick et al[21] postulated that 93% of them were accompanied with gastric type epithelium in the bulb. However, Hara et al[22] demonstrated that only 9.7% to 55.0% (mean 31.8%) of the total surface of the duodenal mucosa taken from ulcer scar areas showing no methylene blue absorption, was occupied by metaplastic cells. We used methylene blue to stain the mucosa in the duodenal bulb to investigate the extent of gastric metaplasia among non-ulcer patients, DU with a mild bulbar deformity and DU with a marked bulbar deformity[19]. The incidence of gastric metaplasia in the duodenal bulb was higher in patients with healed ulcers than in non-ulcer patients. Patients with deformed duodenal bulbs had a higher extent of gastric metaplasia than those without deformed duodenal bulbs[19]. The present study revealed that there were 57.14% of 14 healed type I, 81.25% of 16 healed type II, and 92.87% of 14 healed type III ulcers having grade 1 or 2 gastric metaplasia. In average, there were 72.27% of healed duodenal ulcers to be accompanied with gastric metaplasia. The incidence of gastric metaplasia of healed duodenal ulcers in this series was higher than that reported by Hara et al[22], but definitely lower than that reported by Carrick et al[21]. Because our specimens were taken from the center of ulcer scar, they could exactly represent the condition of regenerating mucosa. On the other hand, our sampling method was similar to that of Hara et al[22], it might be the reason why our data were somewhat close to that of Hara et al[22]. We considered that the difference in the incidence of gastric metaplasia between our study and that reported by Carrick et al[21], might be due to the different biopsy sites.
Metaplastic gastric type epithelium in the duodenum has been regarded as an adaptive defensive response to mucosal injury of any kind, including acid and/or pepsin[20,23,24]. The presence and extent of gastric metaplasia have been correlated with acid secretory capacity in men[20,25], and they have been induced in experimental animals by stimulation of excessive acid secretion[26,27]. Therefore, increased acid load of the duodenum has been considered as the most important determinant of gastric metaplastic changes in duodenal mucosa. However, it should be emphasized that metaplasia is a consequence of any mucosal injury and follows any ulcerative process affecting the duodenum. An increase in both the extent and frequency of gastric mucosa in the duodenal bulb of patients with ulcer diseases would occur due to a duodenal ulcer[20]. The present study revealed that there were 57.14% of regenerating mucosae of healed type I ulcers having evidence of gastric metaplasia, which was statistically higher than that of healthy subjects (57.15% vs 17.5%, P<0.05). Meanwhile, healed type II and type III ulcers had a significantly higher incidence (P<0.01 in each) and a higher degree (P<0.05 in each) of gastric metaplasia than those of healed type I ulcers. The above lines of evidence strongly suggested that presence and degree of gastric metaplasia of regenerating duodenal mucosa were highly correlated with bulbar deformity.
It has been reported that duration of the illness of patients with duodenal ulcer who had a marked deformed bulb was usually more than 6 years or even over 10 years, which was significantly longer than that of ulcer patients with a normal-shaped bulb[13]. Hence, ulcers with a marked deformed bulb would be the most chronic form of duodenal ulcer, and the deformity of duodenal bulb could be regarded as the results of tissue scarring due to periodic relapse and healing of the duodenal ulcer. We considered that the high incidence and degree of gastric metaplasia in healed type II and type III ulcers might be the results of repeated recurrence and healing of the duodenal ulcer, and a deformed bulb with a high degree of gastric metaplasia easily colonized by H pylori, would provide an environment of easy recurrence of the ulcer. Therefore, a vicious cycle of healing and recurrence may exist in this kind of ulcer patients. This may explain why the recurrence of duodenal ulcer was said to be related to deformity of the bulb[13-15], and why the results of our previous study revealed that duodenal ulcers with a normal-shaped bulb had a better chance to be cured and those with a marked deformity of bulb had a high incidence of recurrence or needed surgical intervention[15]. Eradication of H pylori may be the best way to break this vicious cycle and therefore, to prevent repeated recurrence of duodenal ulcers which have a deformity of duodenal bulb. In addition to H pylori eradication, we observed two characteristic conditions in patients with duodenal ulcer recurrence after H pylori eradication: one was a markedly deformed bulb with a high grade metaplasia at the scarring stage, and the other was a change in gastric metaplasia from a high grade to a low grade at the previous ulcer site after ulcer healing.
Results of this study showed that H pylori infection was the most important factor contributing to recurrence of duodenal ulcer. However, both the deformity of bulb and the grade of gastric metaplasia of regenerating duodenal mucosa might also contribute to ulcer recurrence. Because our sample size was not large enough to separate the H pylori-infected and H pylori-eradicated cases, we could not study the influence of deformity of bulb on ulcer recurrence after H pylori eradication. Further study is necessary to clarify this interesting question.
In conclusion, there is a correlation among deformity of duodenal bulb, gastric metaplasia of duodenal regenerating mucosa and recurrence of duodenal ulcer. A more severely deformed duodenal bulb is closely related to a greater extent of gastric metaplasia. Both factors contribute to the recurrence of duodenal ulcer.
Science Editor Wang XL Language Editor Elsevier HK
| 1. | Korman MG, Hetzel DJ, Hansky J, Shearman DJ, Don G. Relapse rate of duodenal ulcer after cessation of long-term cimetidine treatment: a double-blind controlled study. Dig Dis Sci. 1980;25:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Marks IN, Lucke W, Wright JP, Girdwood AH. Ulcer healing and relapse rates after initial treatment with cimetidine or sucralfate. J Clin Gastroenterol. 1981;3:163-165. [PubMed] |
| 3. | Ippoliti A, Elashoff J, Valenzuela J, Cano R, Frankl H, Samloff M, Koretz R. Recurrent ulcer after successful treatment with cimetidine or antacid. Gastroenterology. 1983;85:875-880. [PubMed] |
| 4. | Coghlan JG, Gilligan D, Humphries H, McKenna D, Dooley C, Sweeney E, Keane C, O'Morain C. Campylobacter pylori and recurrence of duodenal ulcers--a 12-month follow-up study. Lancet. 1987;2:1109-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 302] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Marshall BJ, Goodwin CS, Warren JR, Murray R, Blincow E, Blackbourn S, Phillips M, Waters T, Sanderson C. Long-term healing of gastritis and low duodenal ulcer relapse after eradication of Campylobacter pyloridis: a prospective double-blind study. Gastroenterology. 1987;2:1518. |
| 6. | Lambert JR, Borromeo M, Korman MG, Hansky J, Eaves ER. Effect of colloidal bismuth (De-Nol) on healing and relapse of duodenal ulcer - role of Campylobacter pyloridis. Gastroenterology. 1987;92:1489. |
| 7. | Borody T, Cole P, Noonan S, Morgan A, Ossip G, Masey J, Brandl S. Long-term Campylobacter pylori recurrence post-eradication. Gastroenterology. 1988;94:A43. |
| 8. | Tytgat GN, Rauws EA. The role of Campylobacter pylori in gastroduodenal diseases. A "believer"'s point of view. Gastroenterol Clin Biol. 1989;13:118B-121B. [PubMed] |
| 9. | Rauws EA, Tytgat GN. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990;335:1233-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 626] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 10. | Blum AL, Armstrong D, Dammann H, Fischer M, Greiner L, Haase W, Hogeboom-Verdegal A, Liszkay M, Stolte M, Sulser H. The effect of Helicobacter pylori on the healing and relapse of duodenal ulcer. Gastroenterology. 1990;98:A22. |
| 11. | Pan S, Liao CH. The histological maturity of regenerating mucosa of healed duodenal ulcer and ulcer recurrence after treatment with H2-antagonist. Am J Gastroenterol. 1990;85:949-952. [PubMed] |
| 12. | Pan SA, Liao CH, Lien GS, Chen SH. Histological maturity of healed duodenal ulcers and ulcer recurrence after treatment with colloidal bismuth subcitrate or cimetidine. Gastroenterology. 1991;101:1187-1191. [PubMed] |
| 13. | Pan S, Liao CH. An endoscopic study on the duodenal ulcer. An endoscopic classification of the duodenal ulcer and its clinical implications. Taiwan Yi Xue Hui Za Zhi. 1981;80:815-829. [PubMed] |
| 14. | Pan S, Liao CH. Recurrence of duodenal ulcer--an endoscopic study. Taiwan YiXueHui ZaZhi. 1987;86:400-404. [PubMed] |
| 15. | Pan S. Clinical recurrence patterns of duodenal ulcer and deformity of the duodenal bulb. A correlative study. Gastroenterol Endosc. 1987;29:619-625. |
| 16. | Goodwin CS. Duodenal ulcer, Campylobacter pylori, and the "leaking roof" concept. Lancet. 1988;2:1467-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Dixon MF. Helicobacter pylori and peptic ulceration: histopathological aspects. J Gastroenterol Hepatol. 1991;6:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 137] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Fitzgibbons PL, Dooley CP, Cohen H, Appleman MD. Prevalence of gastric metaplasia, inflammation, and Campylobacter pylori in the duodenum of members of a normal population. Am J Clin Pathol. 1988;90:711-714. [PubMed] |
| 19. | Chang CC, Pan S, Lien GS, Chen SH, Cheng CJ, Liu JD, Cheng YS, Suk FM. Investigation of the extent of gastric metaplasia in the duodenal bulb by using methylene blue staining. J Gastroenterol Hepatol. 2001;16:729-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Patrick WJ, Denham D, Forrest AP. Mucous change in the human duodenum: a light and electron microscopic study and correlation with disease and gastric acid secretion. Gut. 1974;15:767-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Carrick J, Lee A, Hazell S, Ralston M, Daskalopoulos G. Campylobacter pylori, duodenal ulcer, and gastric metaplasia: possible role of functional heterotopic tissue in ulcerogenesis. Gut. 1989;30:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 131] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Hara M, Harasawa S, Tani N, Miwa T, Tsutsumi Y. Gastric metaplasia in duodenal ulcer. Histochemical considerations of its pathophysiological significance. Acta Pathol Jpn. 1988;38:1011-1018. [PubMed] |
| 23. | Morrissey SM, Ward PM, Jayaraj AP, Tovey FI, Clark CG. Histochemical changes in mucus in duodenal ulceration. Gut. 1983;24:909-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Gregory MA, Moshal MG, Spitaels JM. Changes in the morphology of villar epithelial cells adjacent to duodenal ulcers during the process of healing. Scand J Gastroenterol. 1982;17:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | James AH. Gastric epithelium in the duodenum. Gut. 1964;5:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Rhodes J. Expermental production of gastric epithelium in the duodenum. Gut. 1964;5:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Tatsuta M, Iishi H, Yamamura H, Yamamoto R, Taniguchi H. Enhancement by tetragastrin of experimental induction of gastric epithelium in the duodenum. Gut. 1989;30:311-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |