INTRODUCTION
Reflux esophagitis (RE) is a common disease entity, with an estimated incidence of 16.3% in Japanese adults[1]. It is well recognized that RE results from excess reflux exposure of esophageal mucosa to acidic gastric juice or bile-containing duodenal contents through an incompetent lower esophageal sphincter[2]. However, acid and bile exposure times, as shown by 24-h monitoring methodologies, greatly overlapped among the diverse gastroesophageal reflux disease (GERD) ranging endoscopically normal-appearing esophageal mucosa to severe RE with complications, meaning that the degree of esophageal mucosal damage might not correlate with the amount of reflux materials[3-6]. Thus, the exact pathophysiological mechanisms of RE are not fully understood.
Recently, several studies have shown that mucosal immune and inflammatory responses, characterized by specific cytokine and chemokine profiles, may underlie the diversity of esophageal phenotypes of GERD[7-11]. Of note, several investigators reported significantly higher expression levels of interleukin 8 (IL-8) messenger ribonucleic acid (mRNA) and protein products in esophageal mucosa of RE patients, compared to subjects with non-inflamed or Barrett’s esophagus[7,8]. In addition, the IL-8 mRNA and protein levels were substantially decreased after lansoprazole therapy[8,10]. The enhanced mucosal IL-8 production paralleled the endoscopic severity of RE[8]. These results indicate that IL-8 is involved in the pathogenesis of RE.
IL-8 mediates its actions via two distinct receptors, CXC receptor 1 (CXCR-1) and CXCR-2[12-14]. Unlike CXCR-1, CXCR-2 is not specific for IL-8 and can bind to other chemokines such as growth-related oncogene α, but it has higher affinity for IL-8 than CXCR-1[15]. In a previous work, we demonstrated that the epithelial cells as well as neutrophils were immunoreactive for IL-8 receptors in esophageal mucosa[10]. Despite such intense interest in the role of IL-8 in GERD, little is known regarding the expression levels of IL-8 receptors in RE. Therefore, we studied the expression of CXCR-1 and CXCR-2 mRNA in the esophageal biopsy tissue of patients with RE using semiquantitative reverse transcriptase polymerase chain reaction (RT-PCR). The implication of their relative mRNA levels in pathological hallmarks of RE was also examined.
MATERIALS AND METHODS
Materials
We studied 26 outpatients who underwent upper gastrointestinal endoscopy for gastroesophageal reflux-related symptoms and were diagnosed as having RE between April 2002, and September 2003. They included 17 men and 9 women, aged between 27 and 80 years (mean 58.7 years). The RE endoscopic findings were classified into grades A, B, C and D according to the Los Angeles (LA) classification system[16]. None of these patients had been treated with nonsteroidal anti-inflammatory drugs, proton pump inhibitors, histamine-2 receptor antagonists, anticholinergic agents, or antibiotics within 4 wk before the present study. Furthermore, patients with severe concomitant diseases, prior esophageal or gastric surgery, peptic ulcer diseases and comorbid conditions that might interfere with esophageal or gastric motility including diabetes mellitus, systemic sclerosis and neurological disorders were excluded. As a control group, we recruited 15 asymptomatic subjects with no hiatal hernia or any lesions in the esophagus, stomach and duodenum at endoscopy for health check-up. The controls included nine men and six women, aged between 39 and 80 years (mean 61.1 years).
In each group, paired biopsy specimens were obtained from the esophageal mucosa, 3 cm above the gastroesophageal junction[8,10,11]. These included one biopsy snap frozen in an ethanol-dry ice mixture and then stored at -80 °C until use for semiquantitative analysis of CXCR-1 and -2 mRNA expression and another for histopathological examination.
Histopathological examination
Paraffin-embedded biopsy specimens were sectioned at 5 µm thickness, and stained with HE. Two independent observers who were blinded to the endoscopic findings and experimental results examined the tissue sections. The histopathological evaluation included basal layer hyperplasia, which was defined as an increase in thickness of the basal layer to more than 15% of the total thickness of esophageal epithelium, elongation of the papillae into the upper one-third of the epithelium, and the presence of intraepithelial neutrophils and eosinophils, based on the criteria described by Ismail-Beigi et al[17]. These criteria allowed sufficient agreement between the two pathologists.
Semiquantitative RT-PCR
Total RNA from the biopsy samples was extracted using a commercial kit according to the instructions provided by the supplier (ISOGEN, Nippon Gene Co., Toyama, Japan). One microgram of total RNA was reverse transcribed into complementary DNA (cDNA) in a volume of 25 μL with MuLV reverse transcriptase and random hexamers (both from PE Applied Biosystems, Warrington, UK).
The target sequence of CXCR-1 mRNA was amplified in 26 cycles, each consisting of 30 s at 94 °C for denaturation, 30 s at 53 °C for annealing and 30 min at 72 °C for extension, followed by a final extension for 5 min at 72 °C with specific primers (forward, 5’-CAGATCCACAGATGTGGGAT-3’ and reverse, 5’-TCCAGCCATTCACCTTGGAG-3’) using a RT-PCR kit (Takara Shuzo Co., Otsu, Japan). Similarly, CXCR-2 mRNA expression was detected under the following conditions: amplification in 28 cycles, each consisting of 30 s at 94 °C for denaturation, 30 s at 60 °C for annealing and 30 min at 72 °C for extension, followed by a final extension for 5 min at 72 °C with specific primers (forward, 5’-AGCTGCTCTTCTGGAGGTGT-3’ and reverse, 5’-TTAGAGAGTAGTGGAAGTGTGC-3’)[18]. Glyceraldehyde-3-phosphate dehydrogenase (G3PDH) gene transcript was amplified in 28 cycles, each consisting of 30 s at 94 °C for denaturation, 30 s at 63 °C for annealing and 30 min at 72 °C for extension, followed by a final extension for 5 min at 72 °C with specific primers (forward, 5’-TGAAGGTCGGAGTCAACGGATTTGGT-3’ and reverse, 5’-CATGTGGGCCATGAGGTCCACCAC-3’), and used as an internal control of the processed RNA for each preparation. A 10-µL aliquot of each PCR product was analyzed by electrophoresis on 2% agarose gel containing ethidium bromide, and the bands were examined under ultraviolet light for the presence of amplified DNA. The density of each band was measured with Lumi-Imager F1 Workstation (Roche Diagnostic Ltd, Tokyo, Japan), and relative mRNA expression level in each sample was expressed as the ratio of CXCR-1 or CXCR-2/G3PDH in the band density, as described previously[19].
Detection of H pylori infection
Helicobacter pylori (H pylori) status was assessed by serology (anti-H pylori Immunoglobulin G antibody, HEL-p TEST, AMRAD Co., Melbourne, Australia), rapid urease test (Helicocheck, Otsuka Pharmaceutical Co., Tokushima, Japan) and histopathology (hematoxylin-eosin and Giemsa staining) using biopsy specimens obtained during endoscopy from the antrum within 2 cm of the pyloric ring and the corpus along the greater curvature. Patients were considered positive for H pylori infection when at least two of these examinations yielded positive results. On the other hand, patients were defined as H pylori negative if all test results were negative.
All samples were obtained with written informed consent of the patients prior to their inclusion in this study, in accordance with the Helsinki Declaration.
Statistical analysis
Statistical analyses were performed using Fisher’s exact, χ2, Student’s t, Mann-Whitney U, and Kruskal-Wallis tests, whenever appropriate. A P value of less than 0.05 was accepted as statistically significant. Data were expressed as mean±SD.
RESULTS
Based on the endoscopic grading of the LA system, cases of RE were classified as grade A (n = 18), B (n = 5) and C (n = 3). None of the RE patients were classified as grade D or had RE-related complications such as stricture or columnar-lined esophagus. The diagnosis of hiatus hernia was established in 15 of the 26 RE patients (57.7%). There were no significant differences in sex, age, body mass index, current tobacco use, alcohol intake, H pylori status and the presence of hiatal hernia among the grades. H pylori infection was detected in 14 patients with RE (53.8%) and in 7 controls (46.7%). There were no significant differences in baseline characteristics between patients with RE and asymptomatic controls.
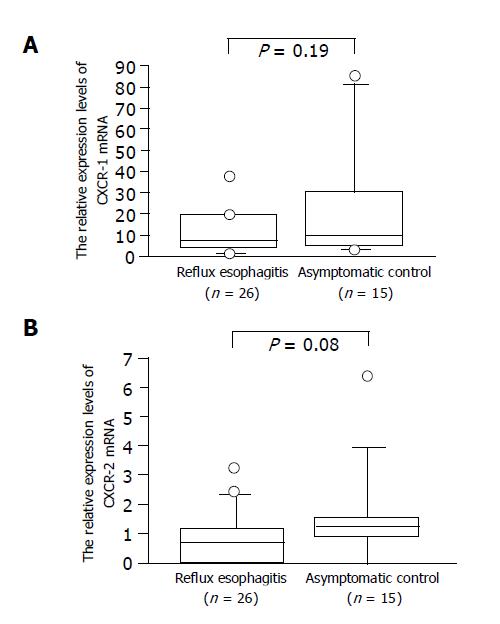
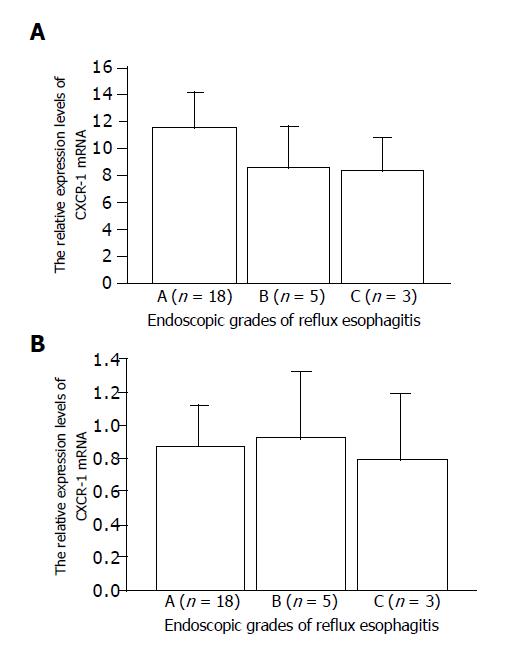
We identified the CXCR-1 and -2 and G3PDH gene-specific products as 257-, 1154- and 983-bp bands, respectively, by RT-PCR, as shown in our previous work (data not shown). The relative CXCR-1 and -2 expression levels in RE patients tended to be lower than those in controls, but the difference was not significant (Figure 1). The relative expression levels of CXCR-1 and CXCR-2 mRNA did not differ among the endoscopic grades (Figure 2).
Figure 1 Relative CXCR-1.
(A) and CXCR-2; (B) mRNA expression levels in patients with RE and asymptomatic controls.
Figure 2 Relative expression levels of CXCR-1.
(A) and CXCR-2; (B) mRNA in terms of endoscopic grades assessed by LA classification.
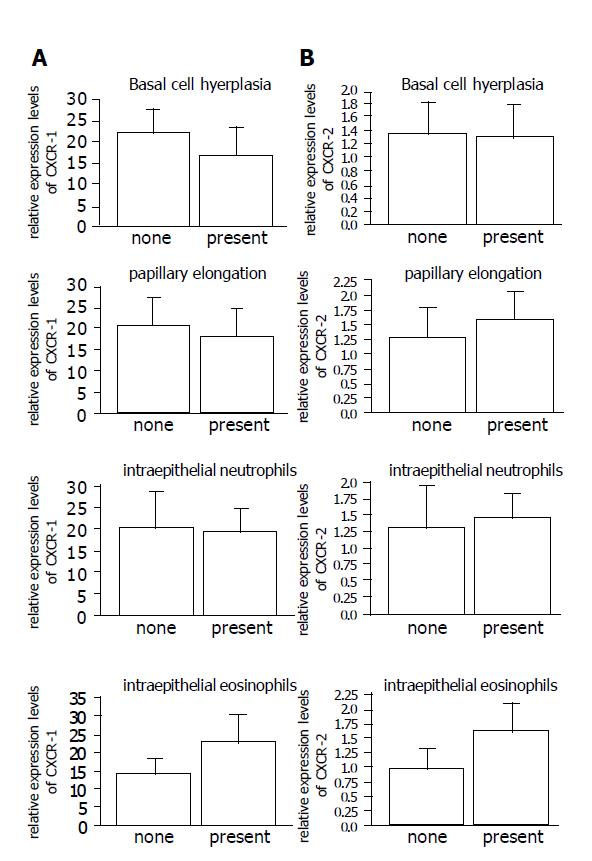
There were no significant differences in the relative CXCR-1 and -2 expression levels in the presence or absence of each histopathological indicator (Figure 3).
Figure 3 The relationship between the relative expression levels of CXCR-1.
(A) and CXCR-2; (B) mRNA and each histopathological hallmarks of RE.
DISCUSSION
IL-8, a representative CXC chemokine, exhibits a potent chemotactic activity for neutrophils[10-12]. In our recent studies, there was a significant relationship between the presence of intraepithelial neutrophils and the expression levels of IL-8 mRNA and protein in patients with GERD[8,10]. We also demonstrated that higher expression levels of IL-8 mRNA and protein were associated with basal layer hyperplasia of esophageal mucosa of patients with GERD[8-10]. It is possible that IL-8, together with other cytokines and growth factors, could contribute to epithelial cell proliferation in GERD[20,21].
There is accumulating evidence that chemokine receptors, as well as chemokines themselves, potentially play an important role in the initiation and progression of immune and inflammatory process[22-26]. In fact, there were several reports on up-regulation of CXCR-1 and/or CXCR-2 expression in various inflammatory conditions including rheumatoid arthritis, ulcerative colitis, and allergic rhinitis[22,23,25]. As IL-8 is regulated in an autocrine manner[27,28], it is tempting to speculate that the interplay of IL-8 and its distinct receptors may contribute to the amplification and protraction of inflammatory response in RE. Unexpectedly, this was not the case, but the relative expression levels of CXCR-1 and -2 mRNA were rather decreased in patients with RE compared to asymptomatic controls. Likewise, there was no significant association between relative mRNA expression levels of the two IL-8 receptors and each histopathological hallmark of RE. Their expression levels did not differ among endoscopic grades of LA classification. Collectively, apart from their functional ligand, IL-8, CXCR-1 and -2 expression levels were unlikely to be implicated in the development and progression of RE.
On the contrary, there have been several reports on down-regulation of IL-8 receptor expression in some inflammatory disorders[18,29,30]. Silvestri et al[31], analyzed a variety of chemokine receptors in the cartilage by immunohistochemistry and RT-PCR. In that study, normal and osteoarthritis-affected cartilage showed moderate to high expression of CXCR-1 and -2, while their expression in samples from inflammatory arthritis ranged from low to absent[30], introducing novel concept of a different role and regulation of the IL-8 ligand-receptor system in diverse disease conditions[32].
In turn, normal esophageal mucosa of asymptomatic controls showed rather higher expression of CXCR-1 and -2 mRNA. Such constitutive nature of their expression might suggest a role of the IL-8 receptors in esophageal tissue homeostasis.
In conclusion, apart from overexpression of IL-8 in RE, the relative expression levels of CXCR-1 and -2 mRNA were rather lower than expected in the affected esophageal mucosa of patients with RE. Further study should be conducted to elucidate such diverse receptor-mediated signaling pathway underlying the pathogenesis of RE.
Science Editor Guo SY Language Editor Elsevier HK