Published online Mar 28, 2005. doi: 10.3748/wjg.v11.i12.1788
Revised: May 28, 2004
Accepted: June 18, 2004
Published online: March 28, 2005
AIM: To characterize p62 expression and define the relationship between p62 expression and cell proliferation in primary carcinomas of the digestive system.
METHODS: p62 expression was characterized in surgically resected tumor specimens from 60 patients with primary carcinomas of the digestive tract (including 22 esophageal carcinomas, 17 gastric carcinomas, and 21 colorectal carcinomas) and 40 patients with hepatocellular carcinoma (HCC) by immunohistochemistry (IHC). The cell proliferation was determined by IHC of Ki-67 in 40 patients with HCC.
RESULTS: Twenty-two cases of esophageal carcinoma were histopathologically diagnosed as squamous cell carcinoma. We combined the gastric and colorectal carcinomas based on the equivalent histology. The 38 tumors in the combined groups, consisted of 17 well-differentiated, eight moderately differentiated, nine poorly differentiated carcinomas, and four mucinous adenocarcinomas. According to the criteria of Edmondson and Steiner, 40 patients with HCC were graded (2 grade I, 17 grade II and 21 grade III). p62 expression in primary carcinomas of the gastrointestinal tract (60/60,100%) was higher than that (27/40, 67.5%) of HCC (P<0.01, χ2 = 19.63). High expression levels of p62 were positively correlated with histological grades in gastric and colorectal carcinomas (P<0.0001) and inversely associated with those in HCC (P = 0.0322). No significant correlations were observed for esophageal carcinomas (P = 0.8246). p62 expression was also detected in the cytoplasm of morphologically normal columnar epithelial cells adjacent to the cancer foci of gastric and colorectal carcinomas. In 40 HCC specimens, the mean Ki-67 labeling index (LI) was (19.6±16.0)%. It was (28.3±18.73)% in 12 cases with high p62 expression (+++), (7.53±14.83)% in 13 cases without p62 expression(-). Patients with a high p62 expression showed a significantly higher level of Ki-67 staining than those without p62 expression (P<0.05, t = 2.069).
CONCLUSION: p62 expression is common in carcinomas of the digestive system and higher in carcinomas of the gastrointestinal tract than in primary HCC. p62 is a cellular differentiation-related protein. Cancer cells with a high p62 expression exhibited highgrowth fractions in HCC.
- Citation: Qian HL, Peng XX, Chen SH, Ye HM, Qiu JH. p62 expression in primary carcinomas of the digestive system. World J Gastroenterol 2005; 11(12): 1788-1792
- URL: https://www.wjgnet.com/1007-9327/full/v11/i12/1788.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i12.1788
p62 is a tumor-related protein identified by Zhang et al[1], using serum auto-antibodies from patients with hepatocellular carcinoma (HCC) to immuno-screen a cDNA expression library in 1999. Of great interest were the findings that during transition from chronic hepatitis and hepatic cirrhosis to HCC, serum auto-antibodies against p62 appeared. These same antibodies were not detected in the pre-malignant condition[2]. Furthermore, the auto-antibodies could be found in many malignant tumors[3,4]. We hypothesize that this antibody response may be stimulated by protein participating in the malignant transformation process[5].
p62 is an insulin-like growth factor II (IGF-II) mRNA binding protein with two types of RNA-binding motifs, two RNA recognition motifs, and four repeats of hnRNP K homology (KH) domains[6]. p62 is a cytoplasmic protein and immunohistochemical analysis of HCC showed that approximately one-third of the patients exhibited high expression of p62 protein in HCC nodules, whereas adjacent non-malignant parenchymal liver cells had no detectable staining. In addition, normal adult liver tissue did not have detectable p62, whereas fetal liver contained immunoreactive p62 in hepatic parenchymal cells[7].
This project sought to determine the following questions: (1) What are the characteristics of p62 expression in carcinomas of the gastrointestinal tract; (2) Are there any differences in p62 expression pattern among carcinomas of the digestive system; and (3) What is the relationship between p62 expression and cell proliferation?
One hundred consecutively resected tumor specimens from the Department of Pathology, the First Clinical Hospital of Medical College of Xiamen University were collected for this study (Table 1). Of these specimens, 22 were esophageal carcinomas (mean age, 57 years; range, 42-73 years), 17 gastric carcinomas (mean age, 57 years; range, 41-76 years), 21 colorectal carcinomas (mean age, 55 years; range, 35-76 years), and 40 HCCs (mean age, 56 years; range, 27-75 years).
Rabbit anti-p62 antibody, p62 antigen and preimmune rabbit (the same one used for anti-p62 antibody production) serum was presented by Eng M. Tan (W.M. Keck Autoimmune Disease Center, Department of Molecular and Experimental Medicine, The Scripps Research Institute, USA). Mouse monoclonal antibody ki-67 (Clone Ki-S5, M7187) and EnVisionTM+ kit (K4010) were purchased from DAKO Corporation. UltraSensitiveTM S-P Kit (Kit 9710) was purchased from Maixin-Bio Company.
A total of 100 formalin-fixed, paraffin-embedded tumor samples were stained immunohistochemically for p62 by EnvisionTM+ kits detection system. At the same time, 40 samples of HCC were also immunostained with ki-67 by labeled streptavidin-biotin method. All sections for both p62 and Ki-67 immunostaining were pretreated with antigen retrieval using citrate buffer at pH6.0.
Four-micron-thick sections were deparaffinized, rehydrated through graded alcohols, transferred into boiled 0.01 mol/L citrate buffer (pH6.0), then boiled again for 10 min, cooled for 20 min, and washed in distilled water for 5 min. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 min. Sections were then incubated with rabbit anti-p62 antibody at a dilution of 1:400 or with mouse monoclonal antibody ki-67 at a dilution of 1:50 for 60 min at room temperature.
Sections for p62 staining were then incubated with peroxidase-labeled polymer for 30 min at room temperature, followed by liquid DAB substrate-chromogen solution for 1 min and counterstained with hematoxylin. Sections for Ki-67 staining were then incubated with biotinylated second antibody and streptavidin conjugated peroxidase for 10 min at room temperature, respectively, followed by liquid DAB substrate-chromogen solution for 2 min. A light Mayer’s hematoxylin was used as a counterstaining.
Negative controls for p62 included replacement of primary antibody to p62 with pre-immune rabbit serum at a dilution of 1:400, and for ki-67 consisted of substituting 0.01 mol/L phosphate buffered saline for the primary antibody. Antibody absorption experiments were performed to confirm the specificity of anti-p62 antibody. Five hundred microliters of diluted anti-p62 antibody was incubated with the 500 μL of diluted p62 protein (0.5 μg/mL) in 37 °C water bath for 2 h, and the absorbed supernatant used as primary antibody.
The immunostained cells for p62 were recorded as positive when cytoplasm of cells exhibited brown staining. The findings were scored according to the numbers of positive cells: -, no or less than 5% cells stained; +, 6% to 25% of cells stained, + +, 26-50% of cells stained, and + + +, more than 50% of cells stained.
For Ki-67 staining, tumor cells were recognized as positive if any nucleus exhibited brown staining. The Ki-67 labeling index (LI) was defined as the percentage of tumor cells displaying nuclear immunoreactivity, and was calculated by counting the number of nucleus-stained tumor cells in 1000 tumor cells in each section. A square grid (10 mm×10 mm) in the ocular lens was used at ×400 in at least five fields to delineate the area in which both the stained and unstained cells were counted. The cases that demonstrated more than 10% positive cells were determined to be positive ones.
The difference in positive rates for p62 between carcinomas of the gastrointestinal tract and HCC was analyzed by χ2 test. The relationship between intensity of p62 immunoreactivity and histological grading was analyzed by Cochran-Mantel-Haenszel test. The difference in mean Ki-67 LI between high (+++) and negative (-) p62 expression was analyzed by Student’s t test. The significance level was set at P<0.05, and all tests were two-sided.
Twenty-two cases of esophageal carcinoma were histopath-ologically diagnosed as squamous cell carcinoma, which were divided into 10 high-, 9 intermediate-, and 3 low-grade ones.
Seventeen cases of gastric carcinoma were histopathologically diagnosed as well-differentiated adenocarcinoma (n = 8), poorly differentiated adenocarcinoma (n = 8), and signet-ring cell carcinoma (n = 1).
Twenty-one cases of colorectal carcinoma were histopathologically diagnosed as well-differentiated (n = 9), moderately differentiated (n = 8), poorly differentiated (n = 1) and mucinous (n = 3) adenocarcinoma.
We combined together the gastric and colorectal carcinomas based on the equivalent histology. The 38 tumors in the combined groups consisted of 17 well-differentiated, 8 moderately differentiated, 9 poorly differentiated, and four mucinous adenocarcinomas.
According to the criteria of Edmondson and Steiner, 40 patients with HCC were graded (two grade I, 17 grade II and 21 grade III).
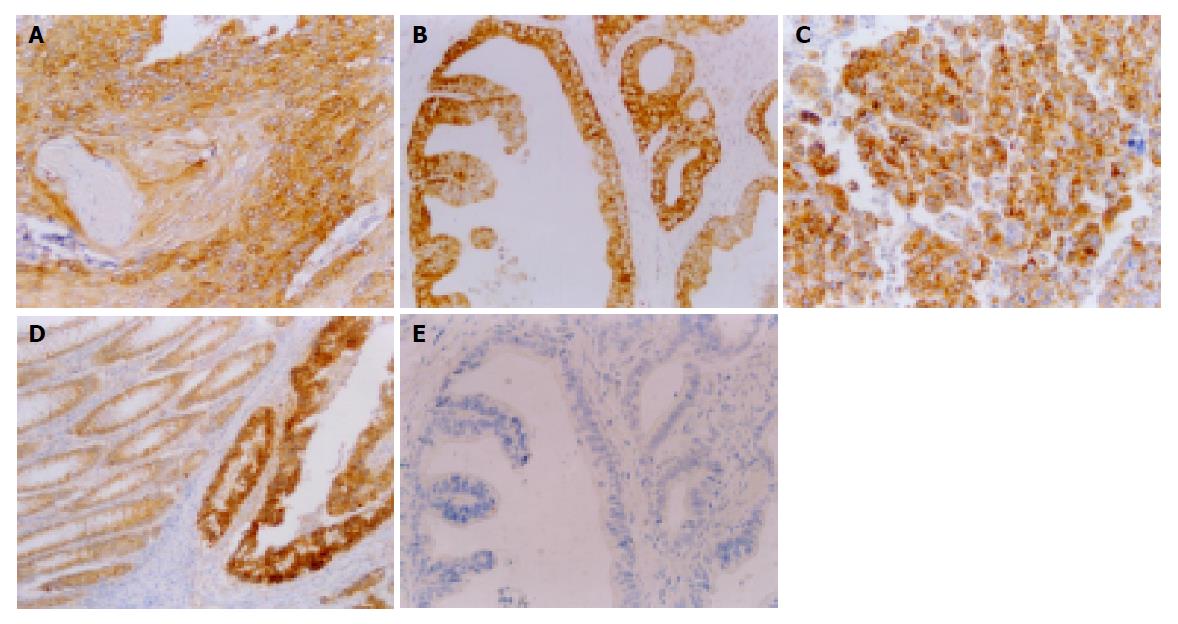
p62 was expressed in the cytoplasm of cancer cells (Figure 1A-C). Immunohistochemical staining revealed that p62 was expressed in all 60 carcinomas of the gastrointestinal tract (100%) and 27 of 40 HCCs (67.5%). There was a significant difference in the expression rate of p62 between carcinomas of the gastrointestinal tract and HCC (χ2 = 19.63, P<0.01) (Table 1).
In carcinomas of the gastrointestinal tract, p62 positive cases were mostly categorized as ++ or +++. Furthermore, in well-differentiated adenocarcinomas, almost all cancer cells uniformly showed strong reactivity to anti-p62 antibody. But in mucinous adenocarcinomas, very weak reactions were detected. In squamous cell carcinoma, the site of keratinization was negative for p62. In HCC, the staining was categorized as + in 8, ++ in 7, +++ in 12 and - in 13 cases. It is worth-mentioning that in contrast to adenocarcinomas, none of the grade I cases of HCC was positive for p62 immunoreactivity, whereas 12 of 17 cases (70%) of grade II, and 15 of 21 cases (71%) of grade III were positive for p62. p62 expression was also detected in the cytoplasm of para-cancerous morphologically normal columnar epithelial cells of gastric and colorectal carcinomas (Figure 1D).
The correlation of intensity of p62 immunoreactivity with histological grading was examined. The Cochran-Mantel-Haenszel test showed that a high expression level of p62 was positively correlated with histological grades in gastric and colorectal carcinomas (P<0.0001) and inversely correlated with histological grades in HCC (P = 0.0322), but no such correlations were found in esophageal carcinoma (P = 0.8246, Table 2).
| Histological grade | Anti-p62 ICH | P | |||
| - | + | ++ | +++ | ||
| Esophageal carcinoma | |||||
| High | 0 | 1 | 5 | 4 | |
| Moderate | 0 | 4 | 1 | 4 | |
| Poor | 0 | 0 | 1 | 2 | 0.8246 |
| Gastric and colorectal carcinoma | |||||
| High + moderate | 0 | 0 | 4 | 21 | |
| Poor + undif. | 0 | 0 | 4 | 5 | |
| MAC | 0 | 4 | 0 | 0 | <0.0001 |
| HCC | |||||
| I | 2 | 0 | 0 | 0 | |
| II | 5 | 6 | 3 | 3 | |
| III | 6 | 2 | 4 | 9 | 0.0322 |
| Mean Ki-67 LI | 7.53±14.84 | 17.89±14.42 | 29.29±16.55 | 28.30±18.73a | |
All cancer cells were negative for pre-immune rabbit sera and anti-p62 antiserum reabsorbed with p62 protein (Figure 1E).
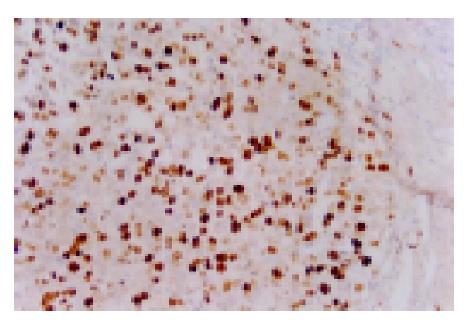
IHC for Ki-67 showed a distinct nuclear staining (Figure 2). In 40 specimens of HCC, Ki-67 LI ranged from 0% to 56.7%, and the mean Ki-67 LI was (19.6±16.0)%. It was (28.30±18.73)% in 12 cases with high p62 expression (+++), (29.29±16.55)% in 7 cases with moderate p62 expression, (17.89±14.42)% in 8 cases with low p62 expression and (7.53±14.84)% in 13 cases without p62 expression (-) (Table 2). To make sure that p62 positive and Ki-67 positive cells were the same cluster of cells in the two consecutive sections, the mean Ki-67 LI was only compared between the high (+++), in which cancer nodules uniformly showed strong reactivity to p62 antibody, and negative (-) p62 expressing tissues. The difference in mean Ki-67 LI between the two groups was statistically significant (t = 3.08, P<0.05). The higher the level of p62 expression was, the higher the Ki-67 LI.
Carcinogenesis is a multistep process involving not only genetic mutation in oncogenes and tumor-suppressor genes but also other complex factors conferring tumorigenic traits. There is growing evidence that a number of intracellular proteins with RNA-binding motifs are associated with cancer[5,8-11]. p62 is an IGF-II mRNA binding protein (IMP). The family of IMP has three members, namely IMP-1, -2, and -3[12]. p62 is identical to IMP-2[7]. p62 has become a highly interesting cancer auto-antigen comparable to p53[5] and has been detected in HCC tissues[7]. Our study revealed that p62 was expressed in esophageal, gastric, colorectal and HCC. The cytoplasmic localization of p62 protein is consistent with its expression in tumor hepatocytes described by Lu et al[7]. The results demonstrated that the expression of p62 was a common phenomenon in carcinomas of the digestive system.
In this study, the frequency of p62 expression in carcinomas of the gastrointestinal tract (100%) was significantly higher than in HCC (67.5%). Furthermore, p62 expression was also detected (100%) in the cytoplasm of para-cancerous non-malignant columnar epithelial cells of gastric and colorectal carcinoma, but not in the adjacent non-malignant parenchymal liver cells of HCC. The expression pattern of p62 in gastric and colorectal carcinoma was similar to that of carcinoembryonic antigen (CEA), which expressed not only in gastric and colorectal carcinoma but also in mucous neck cells and pyloric mucous cells in the stomach and in columnar epithelial cells and goblet cells in the colon[13]. The importance of this finding was that, similar to CEA, p62 should be viewed as a normal adult tissue component of the stomach and colon with retained expression in tumors. In contrast, p62 was aberrantly expressed in HCC. Such different expression pattern indicated a different role of p62 in tumorigenesis of the digestive system.
It was found that the ratio of p62 positive cases in HCC (27/40, 67.5%) was higher than that of a previous study (9/27, 33%) reported by Lu et al[7]. This is not surprising because the assay system and positive criteria used in this study were different from those used in Lu’s study. The DAKO EnvisionTM+ detection system, a highly sensitive system, was used and the cases in which total numbers of p62-expressing cell were more than 5% scored positive in this study. Whereas, the immunofluorescent method was used and the cases in which cancer nodules uniformly showed strong reactivity to p62 antibody scored positive in Lu’s study. If only 12 out of 40 cases with a high expression level (+++) of p62 were defined as positive in this study, the ratio of p62 positive cases was 30% (12 of 40), which is in accordance with that in Lu’s report.
This study highlights the observation that the intensity of p62 expression was associated with the degree of differentiation of tumors. In gastric and colorectal carcinomas, well-differentiated ones showed significantly higher expression levels of p62 than poorly differentiated ones. Inversely, in HCC, poorly differentiated ones exhibited a significantly higher expression level of p62 than well-differentiated ones. p62 expression was involved in cellular differentiation, suggesting that it may be a prognostic indicator.
Ki-67 protein is a nuclear and nucleolar protein, and is closely associated with somatic cell proliferation. Ki-67 antibodies have been used widely for the estimation of the growth fraction of clinical samples of human neoplasm[14,15]. In order to investigate the correlation between p62 and cell proliferation, Ki-67 was analyzed in 40 specimens of HCC in this study. We observed a correlation between immunoreactivity for p62 and Ki-67 LI in HCC. The tumors with a high p62 expression showed a significantly high cell growth fraction determined by the Ki-67 LI, indicating that p62 is one of the factors promoting cellular proliferation. Several studies have demonstrated that the higher the Ki-67 LI, the lower the cellular differentiation of HCC[16-18]. The present study proved the same relationship between Ki-67 LI and cellular differentiation in HCC indirectly, that is, p62 expression was positively associated with Ki-67 LI and inversely associated with cellular differentiation.
What is the role of p62 in tumorigenesis? Lu et al[7], indicated that the role of p62 in tumorigenesis could be regulation of mRNA stability such as IGF-II mRNA. Stabilization of such mRNA increases cell proliferation. Zhang et al, showed that IGF-II was expressed in the cytoplasm of both human hepatoma cell lines and malignant hepatocytes in early experimental HCC tissues. Moorehead et al[19], produced IGF-II transgenic mice and showed that transgenic over-expression of IGF-II in lung epithelium induced lung tumors in 69% of mice older than 18 mo of age. p62 has been closely associated with cell proliferation in the present study, supporting Lu’s hypothesis.
In conclusion, p62 expression is a common phenomenon in carcinomas of the digestive system. p62 should be viewed as a normal adult tissue component of the stomach and colon with retained expression in the tumor and in contrast, it is aberrantly expressed in HCC. p62 is a cellular differentiation-related protein. Cancer cells with a high p62 expression demonstrated high growth fractions in HCC.
We thank Dr. Eng M. Tan (W.M. Keck Autoimmune Disease Center, Department of Molecular and Experimental Medicine, The Scripps Research Institute, USA) for his support and Dr. Yong-Sheng Zong (Department of Pathology, Sun Yat-Sen University of Medical Sciences), Dr Jing-Quan Shi (Institute of Pathology, Southwestern Hospital, Third Military Medical University) and Dr. James H. Resau (Analytical, Cellular and Molecular Microscopy and Laboratory of Microarray, Van Andel Institute, USA) for reviewing our manuscript.
Science Editor Zhu LH Language Editor Elsevier HK
| 1. | Zhang JY, Chan EK, Peng XX, Tan EM. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J Exp Med. 1999;189:1101-1110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 161] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Zhang JY, Zhu W, Imai H, Kiyosawa K, Chan EK, Tan EM. De-novo humoral immune responses to cancer-associated autoantigens during transition from chronic liver disease to hepatocellular carcinoma. Clin Exp Immunol. 2001;125:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Zhang JY, Chan EK, Peng XX, Lu M, Wang X, Mueller F, Tan EM. Autoimmune responses to mRNA binding proteins p62 and Koc in diverse malignancies. Clin Immunol. 2001;100:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Soo Hoo L, Zhang JY, Chan EK. Cloning and characterization of a novel 90 kDa 'companion' auto-antigen of p62 overexpressed in cancer. Oncogene. 2002;21:5006-5015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Tan EM. Autoantibodies as reporters identifying aberrant cellular mechanisms in tumorigenesis. J Clin Invest. 2001;108:1411-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Zhang J, Chan EK. Autoantibodies to IGF-II mRNA binding protein p62 and overexpression of p62 in human hepatocellular carcinoma. Autoimmun Rev. 2002;1:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Lu M, Nakamura RM, Dent ED, Zhang JY, Nielsen FC, Christiansen J, Chan EK, Tan EM. Aberrant expression of fetal RNA-binding protein p62 in liver cancer and liver cirrhosis. Am J Pathol. 2001;159:945-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Ross J, Lemm I, Berberet B. Overexpression of an mRNA-binding protein in human colorectal cancer. Oncogene. 2001;20:6544-6550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Ioannidis P, Trangas T, Dimitriadis E, Samiotaki M, Kyriazoglou I, Tsiapalis CM, Kittas C, Agnantis N, Nielsen FC, Nielsen J. C-MYC and IGF-II mRNA-binding protein (CRD-BP/IMP-1) in benign and malignant mesenchymal tumors. Int J Cancer. 2001;94:480-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Doyle GA, Bourdeau-Heller JM, Coulthard S, Meisner LF, Ross J. Amplification in human breast cancer of a gene encoding a c-myc mRNA-binding protein. Cancer Res. 2000;60:2756-2759. [PubMed] |
| 11. | Gu L, Shigemasa K, Ohama K. Increased expression of IGF II mRNA-binding protein 1 mRNA is associated with an advanced clinical stage and poor prognosis in patients with ovarian cancer. Int J Oncol. 2004;24:671-678. [PubMed] |
| 12. | Nielsen J, Christiansen J, Lykke-Andersen J, Johnsen AH, Wewer UM, Nielsen FC. A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol Cell Biol. 1999;19:1262-1270. [PubMed] |
| 13. | Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 913] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 14. | Endl E, Gerdes J. The Ki-67 protein: fascinating forms and an unknown function. Exp Cell Res. 2000;257:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 271] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 15. | Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 16. | Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385-392. [PubMed] |
| 17. | Nakajima T, Moriguchi M, Mitsumoto Y, Katagishi T, Kimura H, Shintani H, Deguchi T, Okanoue T, Kagawa K, Ashihara T. Simple tumor profile chart based on cell kinetic parameters and histologic grade is useful for estimating the natural growth rate of hepatocellular carcinoma. Hum Pathol. 2002;33:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Aoki T, Inoue S, Imamura H, Fukushima J, Takahashi S, Urano T, Hasegawa K, Ogushi T, Ouchi Y, Makuuchi M. EBAG9/RCAS1 expression in hepatocellular carcinoma: correlation with tumour dedifferentiation and proliferation. Eur J Cancer. 2003;39:1552-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Moorehead RA, Sanchez OH, Baldwin RM, Khokha R. Transgenic overexpression of IGF-II induces spontaneous lung tumors: a model for human lung adenocarcinoma. Oncogene. 2003;22:853-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |