Published online Mar 28, 2005. doi: 10.3748/wjg.v11.i12.1742
Revised: March 10, 2004
Accepted: May 13, 2004
Published online: March 28, 2005
AIM: To investigate the effects of glutamine (GLN)-enriched diets before and GLN-containing total parenteral nutrition (TPN) after sepsis or both on the secretion of cytokines and their mRNA expression levels in splenocytes of rats with septic peritonitis.
METHODS: Rats were assigned to a control group and 4 experimental groups. The control group and experimental groups 1 and 2 were fed a semipurified diet, while experimental groups 3 and 4 had part of the casein replaced by GLN which provided 25% of the total nitrogen. After rats were fed with these diets for 10 d, sepsis was induced by cecal ligation and puncture (CLP), whereas the control group underwent a sham operation, at the same time, an internal jugular vein was cannulated. All rats were maintained on TPN for 3 d. The control group and experimental groups 1 and 3 were infused with conventional TPN, while the TPN in experimental groups 2 and 4 was supplemented with GLN, providing 25% of the total nitrogen in the TPN solution. All rats were kiued 3 d after sham operation or CLP to examine their splenocyte subpopulation distribution and cytokine expression levels.
RESULTS: Most cytokines could not be detected in plasma except for IL-10. No difference in plasma IL-10 was observed among the 5 groups. The IL-2, IL-4, IL-10, and TNF-α mRNA expression levels in splenocytes were significantly higher in experimental groups 2 and 4 than in the control group and group 1. The mRNA expression of IFN-γ was significantly higher in the GLN-supplemented groups than in the control group and experimental group 1. The proportion of CD45Ra+ was increased, while those of CD3+ and CD4+ were decreased in experimental group 1 after CLP was performed. There were no differences in spleen CD3+ lymphocyte distributions between the control and GLN-supplemented groups.
CONCLUSION: GLN supplementation can maintain T-lymphocyte populations in the spleen and significantly enhance the mRNA expression levels of Th1 and Th2 cytokines and TNF-α in the spleen of rats with septic peritonitis.
- Citation: Yeh SL, Lai YN, Shang HF, Lin MT, Chiu WC, Chen WJ. Effects of glutamine supplementation on splenocyte cytokine mRNA expression in rats with septic peritonitis. World J Gastroenterol 2005; 11(12): 1742-1746
- URL: https://www.wjgnet.com/1007-9327/full/v11/i12/1742.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i12.1742
Surgery and trauma induce a generalized state of immunodepression that is correlated with sepsis. Sepsis initiates profound physiologic changes which are characterized by hypermetabolism, altered glucose dynamics, accelerated lipolysis, and alterations in protein metabolism[1]. These metabolic abnormalities occurring during sepsis are mainly resulted from the secretion of cytokines produced by cells of the immune system and a variety of other tissues. Circulating cytokine levels are usually used as markers of injury or infection[2]. However, some cytokines are not detectable at the site of injury or in the systemic circulation due to surgery or sepsis, because the peak time of action varies among cytokines, and many cytokines are bioactive at levels well below the range of detection limit by current immunoassays[3]. Recently, a real-time reverse-transcription polymerase chain reaction (RT-PCR) has been widely used to quantify cytokine profiles in immune cells and inflamed tissues. This technique is very sensitive and accurate and can be performed on very small samples[4].
Total parenteral nutrition (TPN) is commonly used in the treatment of critically ill patients. Sepsis has been shown to reduce mesenteric blood flow, and adversely affect the barrier and metabolic functions of the small intestine[5,6]. Gardiner et al[7], suggested that under a condition of gut-derived sepsis, the parenteral rather than the enteral route had benefits of improving survival in rats. With TPN, adequate caloric and protein intake can be provided. However, a prolonged duration of TPN depresses proinflammatory cytokine production, and impairs the host defense against bacterial infection[8]. Recently, glutamine (GLN) has elicited great interest, because it has been shown to possess numerous useful physiologic properties. The beneficial effects of supplying GLN on metabolic stress conditions include increasing nitrogen retention, preservation of the integrity of the intestinal mucosal and intestinal permeability, maintenance of immunologic functions, and reducing infections[9-12]. We also demonstrated that preventive use of a GLN-enriched enteral diet could enhance peritoneal macrophage phagocytic activity, and that GLN supplementation before or after sepsis could promote proliferation of total lymphocytes in gut-associated lymphoid tissues and enhanced immunoglobulin IgA secretion in septic rats[13,14]. In order to understand the protective mechanisms of GLN, the roles of cytokines in inflammation and immune responses of septic hosts need to be further explored. A study by Wu et al[15], investigated the relation between cytokine mRNA expression levels and organ damage after sepsis. However, to the best of our knowledge, there is no study investigating the effects of GLN administration on mRNA expression levels of Th1 and Th2 cytokines in the spleen of septic rats. Therefore, we administered either a GLN-enriched diet before, GLN-containing TPN after sepsis, or both to investigate the timing of GLN used on splenic cytokine mRNA expression in septic peritonitis. Also, the distribution of splenocyte subpopulations was analyzed to understand the effects of GLN on the phenotype of splenic lymphocytes in septic conditions. We used cecal ligation and puncture (CLP) as the sepsis model in this study, because this model mimics a visceral perforation, and is clinically more relevant than direct intravascular injection of bacteria or pure endotoxin[2,16].
Male Wistar rats aged 8 wk and weighing 200-230 g were used in this study. All rats were housed in temperature- and humidity-controlled rooms and allowed free access to standard rat chow for 1 wk prior to the experiment. The care of the animals followed the guidelines for the care and use of laboratory animals established by the Animal Care Committee of National Taiwan University Hospital, and protocols were approved by the committee.
Rats were randomly assigned to a control group and 4 experimental groups. The control group and experimental groups 1 and 2 were fed a common semipurified diet. Rats in experimental groups 3 and 4 were fed an identical diet except that part of casein was replaced by GLN, which provided 25% of the nitrogen (Table 1). After rats were fed the respective diets for 10 d, sepsis in the experimental groups was induced by CLP, whereas the control group underwent a sham operation. CLP was performed according to the method of Wichterman et al[17]. Briefly, rats were anesthetized with intraperitoneal pentobarbital (50 mg/kg), and the abdomen was opened through a midline incision. The cecum was isolated, and a 3-0 silk ligature was placed around it, ligating the cecum just below the ileocecal valve. The cecum was then punctured twice with an 18-gauge needle and placed back into the abdomen. The abdominal wound was closed in layers. Immediately after the sham or CLP operation, all rats underwent placement of a catheter for TPN infusion. A silicon catheter (Dow Corning, Midland, MI, USA) was inserted into the right internal jugular vein. The distal end of the catheter was tunneled subcutaneously to the back of the neck, and exited through a coiled spring, which was attached to a swivel allowing free mobility of animals inside individual cages. Two milliliters per hour were administered on the first day. Full-strength TPN was given thereafter, and continued for a period of 3 d. The infusion speed was controlled by a Terufusion pump (Model STC-503, Terumo, Tokyo, Japan). The TPN solution without fat was prepared in a laminar flow hood. Sterilized fat emulsions were added to the TPN solution daily just before use. The TPN solution was infused for the entire day at room temperature. All animals were allowed to drink water freely, and no enteral nutrition was administered during the period of TPN. The control group and experimental groups 1 and 3 were infused with conventional TPN. The TPN solutions for experimental groups 2 and 4 were supplemented with GLN, which provided 25% of the total amino acid nitrogen in the TPN solution. TPN provided 280 kcal/kg body weight for the rats. The kcal density of the TPN solution was 1 kcal/mL, and the kcal/nitrogen ratio was 120:1. The TPN solutions were isonitrogenous and identical in nutrient compositions except for the difference in the amino acid content (Table 2). There were 5 groups of rats in this study: control group, GLN not supplemented before or after the sham operation (n = 9); group 1, GLN not supplemented before or after CLP (-/-) (n = 11); group 2, a semipurified diet given before and GLN-containing TPN after CLP (-/+) (n = 9); group 3, a GLN-enriched diet given before and conventional TPN after CLP (+/-) (n = 9); and group 4, a GLN-enriched diet given before and GLN-containing TPN after CLP (+/+) (n = 11).
TPN was continued until kiuing on d 3 after CLP, at which time rats were weighed and anesthetized. After a middle abdominal incision was made, 10 mL PBS was injected intraperitoneally, and peritoneal lavage fluid (PLF) was collected for nitric oxide (NO) measurement. Splenocytes were obtained by mechanical disruption of the spleen with a spatula on a stainless steel mesh. Cell suspensions were passed through a sterile nylon mesh to remove debris. RBCs were lysed by sterile distilled water for 15 s, and immediately neutralized to isotonic cell suspensions. After being washed with PBS thrics (300 r/min centrifugation for 5 min), splenocytes were resuspended in RPMI-1640 with antibiotics and fetal calf serum. The number of isolated splenocytes was determined by a hemacytometric count using the trypan blue dye exclusion method.
Plasma cytokine immunoassay IL-2, IL-4, IL-10, and IFN-γ concentrations in plasma were determined by commercially available ELISA kits (Amersham Pharmacia Biotech, Buckinghamshire, UK).
Determination of NO3-/NO2- in peritoneal lavage fluid NO was highly unstable in solution and could not readily be assayed. However, NO was converted to stable nitrite and nitrate ions in aqueous solution. After conversion of nitrate to nitrite using nitrate reductase, nitrite concentrations were measured using the Griess reagent. NO3-/NO2- concentrations in PLF were determined with a commercial kit (Assay Designs, Ann Arbor, MI, USA). Procedures were described in the manufacturer’s instructions.
Lymphocyte subpopulations in the spleen Flow cytometry was used to determine the proportions of CD45Ra, CD3, CD4, and CD8 from splenocytes. Cells at 105 were suspended in 100 μL HBSS containing fluorescein-conjugated mouse anti-rat CD3 (Serotec, Oxford, UK) and phycoerythrin-conjugated mouse anti-rat CD45Ra (Serotec) to distinguish T and B cells, respectively. Fluorescein-conjugated mouse anti-rat CD8 and phycoerythrin-conjugated mouse anti-rat CD4 (Serotec) were used to identify T helper cells and cytotoxic T lymphocytes, respectively. After being stained for 15 min, 1 mL red blood cell (RBC) lysing buffer (Serotec) was added to lyse the RBCs and to fix the stained lymphocytes. Fluorescence data were collected on 5×104 viable cells and analyzed by flow cytometry (Coulter, Miami, FL, USA).
Real-time reverse-transcription polymerase chain reaction (RT-PCR) method The primers of cytokines (IL-2, IL-4, IL-10, TNF-α, and IFN-β) and the housekeeping gene (18S rRNA) were purchased from Applied Biosystems (Foster City, CA, USA). Total RNA from rat spleens was isolated using the TRIzol reagent according to the manufacturer’s protocol. RNA was reverse-transcribed using the reverse transcript system (Frementas, Vilnius, Lithuania). Briefly, 20 μL water containing 2 μg RNA was mixed with 1 μL oligo (dT) primer (0.5 μg/μL) and incubated for 5 min at 70 °C. To the mixture, 4 μL 5× RT-buffer, 2 μL dNTP (10 mmol/L), 1 μL RNase inhibitor, and 1 μL MultiScribe-RT (200u/μL) were added and incubated at 37 °C for 5 min, then at 42 °C for 60 min. The reaction was stopped by heating the samples for 10 min to 70 °C. cDNA was used for the real-time PCR assay performed with an ABI 7700 sequence detection system (PE Applied Biosystems, Foster City, CA) according to supplied guidelines. PCR reaction for IL-2, IL-4, and IL-10 was carried out using a TaqMan PCR kit (Applied Biosystems).
Data were expressed as mean±SD. Differences among groups were analyzed by one-way ANOVA using Fisher’s test. P<0.05 was considered statistically significant.
Plasma levels of IL-2, IL-4, and IFN-γ could not be detected across groups 3 d after CLP. There were no differences in plasma IL-10 concentrations among all groups (data not shown).
The NO3-/NO2- concentration in group 4 (+/+) was significantly higher than that in the control group (P<0.05), and showed no difference from the other experimental groups (control, 11.2±9.1 μmol/L; group 1, 17.4±14.7 μmol/L; group 2, 15.6±11.2 μmol/L; group 3, 21.5±14.4 μmol/L; group 4, 28.1±16.9 μmol/L).
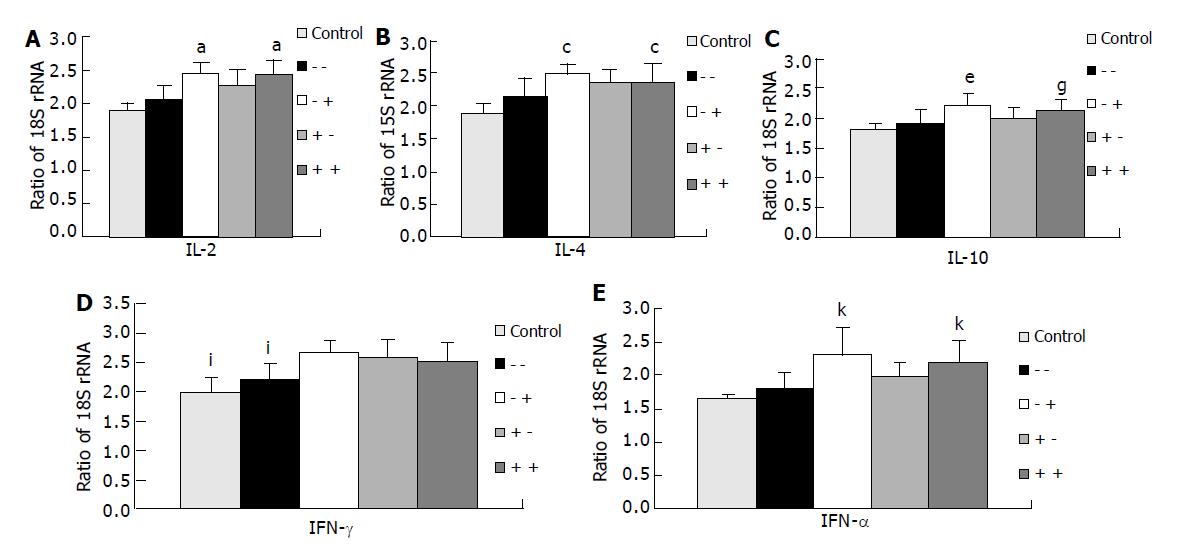
IL-2, IL-4, IL-10, and TNF-α mRNA expression levels in splenocytes were significantly higher in groups 2 (-/+) and 4 (+/+) than in the control group and group 1 (-/-). IL-2 and IL-4 mRNA expressions in group 3 (+/-) were higher than in the control group, but showed no differences from those in group 1 (-/-). There were no differences in IL-10 and TNF-α mRNA expression levels between the control group and experimental groups 1 and 3. mRNA expression levels of IFN-γ were significantly higher in the GLN-supplemented groups (groups 2, 3, and 4) than in the control group and group 1 (Figure 1).
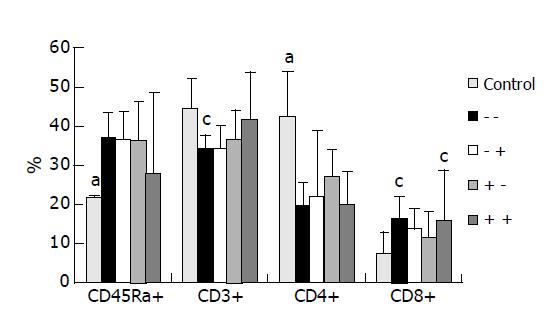
Proportions of CD45Ra+ in splenocytes were significantly higher, whereas CD4+ was significantly lower in the experimental groups than in the control group. No significant differences in CD45Ra+ or CD4+ distributions were observed among the 4 experimental groups. The proportions of CD3+ in group 1 (-/-) were significantly lower than in the control group, whereas no differences were observed among the control and Gln supplemented groups (Figure 2).
In this study, we administered TPN to rats for 3 d and then kiued them, because in a preliminary study, we found that the total number of Peyer’s patches on the serosal side of the intestine was much greater on the 3rd d than on any other days after CLP. This is consistent with the observation that the severity of infection and mortality were the highest at this time point in septic peritonitis[17].
Analysis of cytokine profiles plays a central part in the characterization of disease-related inflammatory pathways and the identification of functional properties of immune cell subpopulations. In this study, we were unable to detect plasma IL-2, IL-4, or IFN-γ on d 3 after CLP. This result is consistent with our previous report that plasma IL-2 and IFN-γ were not detectable 24 h after CLP[18]. Cruickshank et al[19], also reported that plasma IL-1, TNF, and IFN-γ were rarely detected in the plasma of injured patients. In order to understand the possible roles of GLN in systemic cytokine expression after sepsis, expression levels of IL-2, IFN-γ, IL-4, IL-10, and TNF-α mRNA in the spleen were measured. IL-2 and IFN-γ are produced by Th1 lymphocytes. Th1 cytokines could enhance cell-mediated immunity. A predominant Th1 effect could result in activation of macrophages and T lymphocytes, particularly in cytotoxic and delayed-hypersensitive cells. Th2 cytokines, including IL-4 and IL-10, enhance humoral immunity. A predominant Th2 effect results in activation of B lymphocytes and upregulation of IgG1, IgA, and IgE production and mucosal immunity[20]. The effects of Th1 or Th2 lymphocytes are counter-regulatory. In this study, we used real-time RT-PCR to quantify cytokine mRNA expressions in the spleen. Real-time RT-PCR has been proven to have a good correlation in transcription levels with competitive RT-PCR, and to be a sensitive and rapid tool for quantifying mRNA expression[4]. The results demonstrated that mRNA expression levels of IL-2, IFN-γ, IL-4, and IL-10 in splenocytes were significantly higher in GLN-supplemented groups than in the control group. This finding indicates that GLN enhanced both Th1 and Th2 cytokine mRNA expression levels in septic conditions, and GLN administered after CLP had a greater effect than before CLP on cytokine mRNA expression.
In this study, we found that the splenocyte proportion of CD45Ra+ was increased, while those of CD3 and CD4 were decreased in group 1 after CLP was performed. These findings suggest that cellular immunity was suppressed and humoral immunity was enhanced under the condition of extracellular infection. Although GLN supplementation seemed to have no effect on the splenic total B (CD45Ra+) lymphocyte distribution in this study, our previous report with the same study design showed that GLN supplementation promoted plasma and intestinal IgA secretion in septic rats[13]. This is consistent with the increased IL-4, IL-10 mRNA expression levels in the present study. A study by Fukatsu et al[21], revealed that TPN could decrease IL-4 and IL-10 mRNA expression levels in lipopolysaccharide-stimulated intestinal laminar propria cells, but GLN supplementation preserved the expression and maintained the intestinal IgA secretion.
IL-2 has effects principally on promotion of growth and differentiation of T lymphocytes. IFN-γ plays a major regulatory role in the macrophage antimicrobial mechanism. TNF-α is a proinflammatory cytokine that could activate macrophages[20]. In this study we found that groups with GLN supplementation after CLP had significantly higher IL-2, IFN-γ, and TNF-α mRNA expression levels than the control group and the group without GLN. These findings are consistent with the results that GLN supplementation helped maintain total T lymphocyte populations in the spleen and enhanced peritoneal macrophage phagocytotic activity as previously described[13]. Moreover, we also observed that the NO3-/NO2- concentration was significantly higher in group 4 (+/+) than in the control group. NO is a simple and unstable free radical produced in large quantities during host defense and immunologic reactions. Because it has cytotoxic properties and is generated by activated macrophages, it has been considered to play a role in nonspecific immunity[22]. It is possible that macrophages are activated after TNF-α and IFN-γ expression which may consequently promote the release of NO, thus enhancing the phagocytic activity of peritoneal macrophages.
In summary, this study showed that GLN administration, especially that administered after CLP, significantly enhanced the mRNA expression levels of Th1 and Th2 cytokines. The mRNA expression of proinflammatory cytokine, TNF-α, was also increased when GLN was administered. Although the effects of Th1 and Th2 lymphocytes are counter-regulatory, cytokine mRNA expression and protein secretion may be regulated by different mechanisms in various tissues and organs, and the influence of the ultimate immune response on specific tissues or organs may vary. Whether there are intracellular factors which regulate the post-transcriptional expression of these cytokines requires further investigation.
| 1. | Souba WW, Herskowitz K, Klimberg VS, Salloum RM, Plumley DA, Flynn TC, Copeland EM. The effects of sepsis and endotoxemia on gut glutamine metabolism. Ann Surg. 1990;211:543-549; discussion 549-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Ertel W, Morrison MH, Wang P, Ba ZF, Ayala A, Chaudry IH. The complex pattern of cytokines in sepsis. Association between prostaglandins, cachectin, and interleukins. Ann Surg. 1991;214:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 183] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Fong Y, Moldawer LL, Shires GT, Lowry SF. The biologic characteristics of cytokines and their implication in surgical injury. Surg Gynecol Obstet. 1990;170:363-378. [PubMed] |
| 4. | Blaschke V, Reich K, Blaschke S, Zipprich S, Neumann C. Rapid quantitation of proinflammatory and chemoattractant cytokine expression in small tissue samples and monocyte-derived dendritic cells: validation of a new real-time RT-PCR technology. J Immunol Methods. 2000;246:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Navaratnam RL, Morris SE, Traber DL, Flynn J, Woodson L, Linares H, Herndon DN. Endotoxin (LPS) increases mesenteric vascular resistance (MVR) and bacterial translocation (BT). J Trauma. 1990;30:1104-1113; discussion 1113-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Gardiner K, Barbul A. Intestinal amino acid absorption during sepsis. JPEN J Parenter Enteral Nutr. 1993;17:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Gardiner KR, Gardiner RE, Barbul A. Reduced intestinal absorption of arginine during sepsis. Crit Care Med. 1995;23:1227-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Okada Y, Papp E, Klein NJ, Pierro A. Total parenteral nutrition directly impairs cytokine production after bacterial challenge. J Pediatr Surg. 1999;34:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Wilmore DW. The effect of glutamine supplementation in patients following elective surgery and accidental injury. J Nutr. 2001;131:2543S-2549S; discussion 2550S-2551S. [PubMed] |
| 10. | Stehle P, Zander J, Mertes N, Albers S, Puchstein C, Lawin P, Fürst P. Effect of parenteral glutamine peptide supplements on muscle glutamine loss and nitrogen balance after major surgery. Lancet. 1989;1:231-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 268] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | van der Hulst RR, van Kreel BK, von Meyenfeldt MF, Brummer RJ, Arends JW, Deutz NE, Soeters PB. Glutamine and the preservation of gut integrity. Lancet. 1993;341:1363-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 446] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 12. | Calder PC. Glutamine and the immune system. Clin Nutr. 1994;13:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Lai YN, Yeh SL, Lin MT, Shang HF, Yeh CL, Chen WJ. Glutamine supplementation enhances mucosal immunity in rats with Gut-Derived sepsis. Nutrition. 2004;20:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Yeh SL, Lai YN, Shang HF, Lin MT, Chen WJ. Effects of glutamine supplementation on innate immune response in rats with gut-derived sepsis. Br J Nutr. 2004;91:423-429. [PubMed] |
| 15. | Wu RQ, Xu YX, Song XH, Chen LJ, Meng XJ. Relationship between cytokine mRNA expression and organ damage following cecal ligation and puncture. World J Gastroenterol. 2002;8:131-134. [PubMed] |
| 16. | Foëx BA, Shelly MP. The cytokine response to critical illness. J Accid Emerg Med. 1996;13:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29:189-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1078] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 18. | Yeh SL, Yeh CL, Lin MT, Lo PN, Chen WJ. Effects of glutamine-supplemented total parenteral nutrition on cytokine production and T cell population in septic rats. JPEN J Parenter Enteral Nutr. 2001;25:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Cruickshank AM, Fraser WD, Burns HJ, Van Damme J, Shenkin A. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci (Lond). 1990;79:161-165. [PubMed] |
| 20. | DiPiro JT. Cytokine networks with infection: mycobacterial infections, leishmaniasis, human immunodeficiency virus infection, and sepsis. Pharmacotherapy. 1997;17:205-223. [PubMed] |
| 21. | Fukatsu K, Kudsk KA, Zarzaur BL, Wu Y, Hanna MK, DeWitt RC. TPN decreases IL-4 and IL-10 mRNA expression in lipopolysaccharide stimulated intestinal lamina propria cells but glutamine supplementation preserves the expression. Shock. 2001;15:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4289] [Cited by in RCA: 4165] [Article Influence: 130.2] [Reference Citation Analysis (0)] |