INTRODUCTION
Glutamate is the major excitatory neurotransmitter in the mammalian central neuronal system (CNS) and is responsible for most fast synaptic neurotransmission[1]. Glutamate signaling in the neuronal tissues has now been widely accepted after the successful identification of glutamatergic transmission system in the CNS. The neurotransmitter is packaged into vesicles in a presynaptic cell after being synthesized or transported into the cell to ensure its high concentration. Upon stimulation, the neurotransmitter diffuses across the synaptic cleft and binds to specific postsynaptic receptors, which activates the receptors and induces cascades of intracellular activity in the postsynaptic cell. Once the stimulation is withdrawn, the neurotransmitter is taken up by the transporter on the plasma membrane of presynaptic cells. Excess intracellular glutamate will be packed into the synaptic vesicle by the vesicular glutamate transporter (VGLUT).
The glutamatergic system consists of glutamate receptor, VGLUT and plasma glutamate transporter[1]. Re-uptake of the released glutamate is mediated by two transport systems. One is high-affinity Na+-dependent carrier located in the plasma membrane[2], and the other is a low-affinity Na+-independent transport system located in the synaptic vesicular membrane[3]. There are two major groups of glutamate receptor: ionotropic glutamate-gated ion channels and G-protein-coupled metabotropic (mGlu) receptors. mGlu receptors are located pre- and postsynaptically, and glutamate acting via mGlu receptors can exert not only an excitatory but also an inhibitory action. Eight mGlu receptors have been cloned and are classified into three groups. Group I (mGlu1 and 5) activate phospholipase C through coupling to the Gq class of G protein. Group II (mGlu2 and 3) and group III (mGlu 4, 6, 7, 8) inhibit cAMP formation through coupling to the Gi/Go class of G protein, reduce Ca2+ currents and presynaptically inhibit the release of glutamate or other neurotransmitters[4]. There are three major ionotropic receptor subtypes named according to their most selective agonists: N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors[5]. The latter two are referred as non-NMDA receptors. All three glutamate receptors are ligand-gated cation channels and are permeable to Ca2+ and/or Na+. Kainate receptors are insensitive to AMPA while AMPA receptors exhibit a low affinity for kainate[5].
Comparing with CNS, the knowledge of glutamate signaling in enteric nervous system (ENS) and non-neuronal digestive tissue is not fully understood. There is compelling evidence for the expression and function of glutamate as a signaling molecule in the digestive system[1]. Multiple glutamate receptors have also been found in the ENS and pancreas[6]. More recently, several groups have shown that the two VGLUTs, VGLUT1 and VGLUT2, are both present in ENS and pancreatic tissue[7] or clonal islet cells[8]. These findings suggested the existence of glutamatergic system in the digestive system. As the first step for the glutamate packaging, VGLUT plays a unique role in the glutamatergic system. In this article, we briefly summarize the recent studies on the molecular physiology of this transporter in the digestive system.
FUNCTIONAL ROLES OF GLUTAMATE IN THE DIGESTIVE SYSTEM
The most recent research on the functional role of glutamate in the digestive system is focused on the ENS of gastrointestinal tract and endocrine pancreas. In the ENS of the gut, the whole glutamatergic system including storage vesicles, neuronal uptake system and receptors has been located[6]. Glutamate has been shown to modulate histamine-induced acid secretion in the rat stomach[9] and the contractility in the gastric fundus, jejunum, ileum and large intestine[10,11]. Neurons that contain glutamate and express VGLUT2 are also present in the ENS[12]. Enteric neurons release glutamate in a Ca2+-dependent manner and activation of both ionotropic and group I mGlu receptors has been associated with the excitation of enteric neurons[6]. Using patch-clamp recording method, group II mGlu receptors were expressed in the gut and these receptors are pertussis toxin sensitive and negatively coupled to N-type Ca2+ channels[13]. More recently it was found that mGluR8, which belong to group III mGlu receptor, is abundant in the submucosal and myenteric plexus in which it is localized to both pre- and postsynaptic elements. Activation of mGluR8 results in receptor internalization as well as inhibition of forskolin-induced cAMP formation in enteric ganglia[14].
Pancreas is another organ in the digestive system and glutamate signaling has great impact on its function. Pancreatic islets of Langerhans, found dispersed throughout the exocrine pancreas, are composed of four major cell types as follows: the insulin-secreting β cell, the glucagons-secreting α cell, the pancreatic polypeptide-secreting PP cell, and the somatostatin-secreting δ cell. They are self-contained miniature organs responsible for hormones insulin, glucagon, somatostatin, and pancreatic polypeptide. Control of hormone release involves complex interactions between circulating fuels and hormones, autocrine and paracrine regulation, and neuronal input. However, the final common pathway to glucagons and insulin release appears to be the electrical activity of individual α or β cells. In the consensus model of glucose-stimulated insulin secretion, ATP is generated by mitochondrial metabolism, promoting closure of ATP-sensitive potassium channels, which depolarizes the plasma membrane. Subsequently, opening of voltage-sensitive Ca2+ channels increases the cytosolic Ca2+ concentration ([Ca2+]c) which constitutes the main trigger initiating insulin exocytosis[15,16]. Nevertheless, Ca2+ signal alone is not sufficient for sustained secretion. Ion channels of islet cell can be regulated by intracellular second messengers, G-proteins, and energy levels, but very few of them respond directly to extracellular stimuli. One mechanism for rapidly translating extracellular chemical signals into electrical signals is through ligand-gated ion channels. γ-amino butyric acid (GABA)-α receptors have been observed in many pancreatic islet cells. Activation of these receptors inhibits glucagons secretion[17] and depolarizes some types of cell lines[16].
Recently, glutamate has been found to act as an intracellular messenger in glucose-induced insulin exocytosis in pancreatic β cell[18-20] and is involved in glucagon exocytosis in pancreatic α cells[18]. Glutamate modulates exocytosis in pancreatic β cells and is involved in glucagon exocytosis through glutamatergic systems including input, output, and termination of glutamate signals[21]. Glucose also increases the cellular glutamate content in INS-1 cells and human islets[19]. In rat islets, Tamarit-Rodriguez et al[22] reported that glutamate was the only amino acid out of 12 that increased during glucose stimulation. Oral or intravenous glutamate can also increase insulin secretion and glucose tolerance in vivo[23]. In addition, overexpression of glutamate decarboxylase (GAD65) in INS-1E cells[24] decreased cellular glutamate level and subsequently reduced glucose-stimulated insulin secretion in pancreatic β cells. Although the physiological role of the glutamatergic system in the islets is not fully understood at present, glutamate may regulate the secretion of insulin and glucagon by way of its binding to the receptors in α and β cells[18,20].
Mechanisms whereby glutamate receptor activation may control hormone secretion from pancreas or preparations of pancreatic cells have recently been described. Bertrand et al[20], showed that glutamate potentiated glucose-stimulated secretion of insulin from perfused pancreas via actions at α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)-type glutamate receptors. Interestingly, AMPA receptor agonists were also shown to increase glucagons secretion from the same preparation. Such observations raise important questions about the role of glutamate receptors in regulating hormone secretion from islet cells. It has been suggested that the multiple, complex consequences of glutamate on islet physiology is via actions of different glutamate receptors expressed in these cells[25]. These studies suggested that the glutamatergic systems in pancreas, islets could play an important role in controlling insulin and glucagon release.
MOLECULAR IDENTIFICATION AND CHARACTERIZATION OF VESICULAR GLUTAMATE TRANSPORTERS
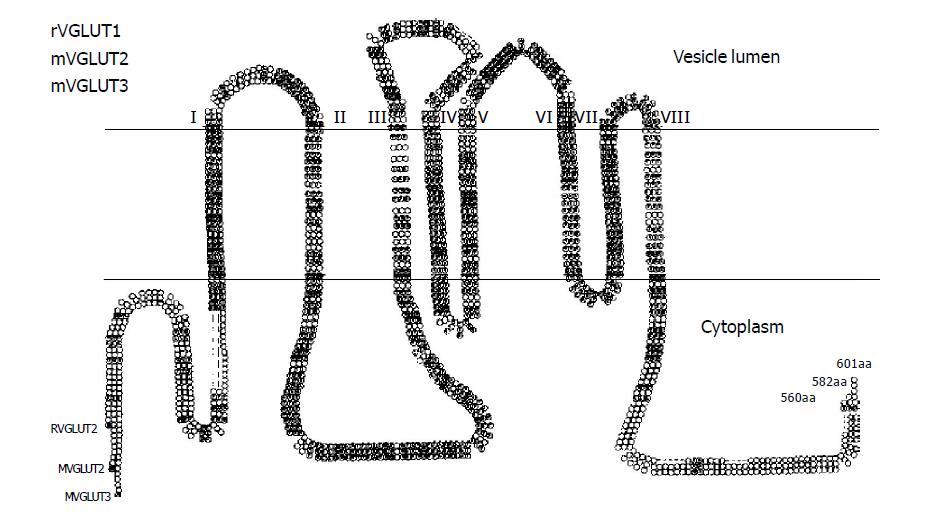
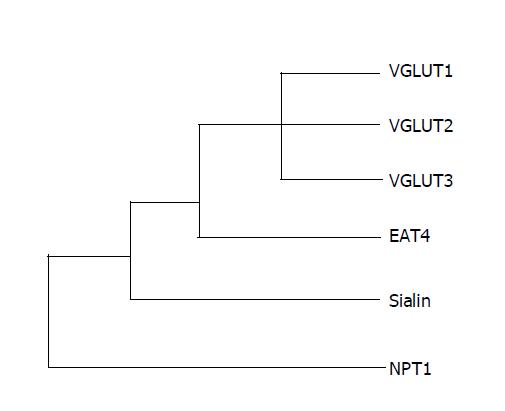
VGLUTs play an essential role in glutamate signal output through vesicular storage of glutamate[26]. Currently, three VGLUTs, VGLUT1[27,28], VGLUT2[29-31], VGLUT3[32-34], have been cloned and functionally characterized. These transporters are highly conserved and have the same predicted topology with 10 putative transmembrane domains as shown in Figure 1. Construction of a phylogenic tree based on the amino acid sequences of related proteins indicates that mouse VGLUTs form a small family that is closely related to Caenorhabditis elegans EAT-4, human sialin, and mouse sodium-dependent phosphate cotransporter 1 (NPT1) (Figure 2). VGLUTs also share similar functional properties, such as ATP dependence, chloride dependence, and substrate specificity.
Figure 1 Predicted secondary structure of VGLUTs.
Primary amino acid sequence and predicted secondary structure of rat VGLUT1 (GenBank accession number NM053859), mouse VGLUT2 (GenBank accession number AF324864), and mouse VGLUT3 (GenBank accession number NM182959). Eight putative transmembrane domains (TMDI–VIII) are indicated. Letter circle indicates amino acids conserved among all three members.
Figure 2 Phylogenic tree of VGLUTs superfamily.
Dendrogram showing the relationship among rat VGLUT1 (GenBank accession number NM053859), mouse VGLUT2 (AF324864), mouse VGLUT3 (NM182959), C. elegans EAT-4 (AF095787), human sialin (AJ387747), and rabbit NPT1 (NM005074).
VGLUT1 was initially cloned as a brain-specific Na+-dependent inorganic phosphate (Pi) cotransporter (BNPI) in 1994[27], and was recently characterized as the first VGLUT[35,36]. The VGLUT1 cDNA encodes a 560-amino acid protein with 8-10 putative transmembrane domains. The VGLUT1 mRNA is expressed predominantly in brain, and especially enriched in the cerebral cortex, hippocampus, and cerebellum[27-30]. In pancreatic islets, VGLUT1 is expressed in pancreatic polypeptide-containing F cells and glucagons secretory α cells[7,37] and clonal β cells[30].
Shortly after the first VGLUT was identified, we and another group cloned VGLUT2, the second isoform of the family, from different species, human, mouse and rat. VGLUT2 has all major functional characteristics of a synaptic VGLUT like VGLUT1, including ATP dependence, chloride stimulation, substrate specificity, and substrate affinity. The human VGLUT2 showed 82% amino acid identity and 92% similarity to VGLUT1. In the CNS, VGLUT2 is highly expressed in medulla, substantia nigra, subthalamic nucleus, and thalamus. Recent studies showed that VGLUT2 is also expressed in the digestive tissue including ENS, stomach, intestine and pancreas. By using RT-PCR and specific antibody, VGLUT2 mRNA and protein are expressed in the cultured α and β cells[30,31]. Hayashi et al[29] also suggested that VGLUT2 is present in pancreatic polypeptide-containing secretory granules in F cells in the islets of Langerhans VGLUT2 and clonal α cells. In stomach VGLUT2 is abundant in the antrum and pylorus and is present in a subset of pancreatic polypeptide-containing cells. VGLUT2 is also abundant in the ileum and is co-localized with glucagon-like immunoreactive peptide and polypeptide YY[27].
VGLUT3 is the third isoform of the VGLUT family that has been cloned very recently[32-34]. In central nervous system, it shows more restricted expression and is present in both excitatory and inhibitory neurons, as well as cholinergic neurons, monoamine neurons, and glia. VGLUT3 is also expressed in liver and kidney[34], which suggests that VGLUT3 functions as a component of peripheral glutamatergic system. VGLUT3 has not been reported in the digestive system. Further studies, particularly in cellular expression and subcellular localization of VGLUT3, will elucidate the potential roles of VGLUT3 in the digestive system.
FUNCTIONAL CHARACTERISTICS OF VESICULAR GLUTAMATE TRANSPORTER IN THE DIGESTIVE SYSTEM
Functional characterization of VGLUT was initially studied in the neurons and some endocrine cells. Synaptic vesicles and microvesicles, enclosed in endocrine cells like pinealocytes, possess an active glutamate-specific transporter that is dependent on the extravesicular Cl- concentration, on an electrochemical proton gradient across the vesicle membrane[38-41] and on the temperature[39]. The dependence of glutamate uptake on ATP-generated proton electrochemical potential was analyzed in a highly purified preparation of synaptic vesicles from rat brain[42]. VGLUT processes depend on the proton electrochemical gradient (ρµH+) generated by a Mg2+-activated vacuolar H+-ATPase (V-ATPase) on the vesicular membrane[43]. When protons are pumped into the vesicular lumen, a proton gradient (ρpH) and a membrane potential (ρφ) occur across the membrane to form ρµH+, which favors the exchange of luminal protons for cytoplasmic transmitter[38,39,42].
Although glutamate signaling has important impact on hormones release from the cells of pancreatic islets, and evidences strongly suggested that pancreatic islets have their own glutamatergic system, functional characterization of VGLUT in pancreatic cells was not determined. By using cultured pancreatic α cells (αTC1-9 and human HPAC) and β cells (rat RIN-m and βTC-6), we have recently functionally characterized VGLUT in these cells[8]. VGLUT in αTC1-9 and βTC-6 cells is ATP dependent, as removal of ATP abolished the uptake. DCCD and bafilomycin A1, inhibitors for vacuolar Mg2+-ATPase, dramatically inhibited glutamate uptake. The plasma membrane and mitochondrial ATPase inhibitors, oligomycin B and ouabain, had no effect on glutamate uptake in both cells. Vesicular transporter recognized only glutamate, but not other amino acid, including aspartate, GABA.
Another feature of the VGLUTs that segregates it from other neurotransmitter transporters is the marked stimulation seen in the presence of low concentrations of chloride[30,43-47]. At low concentrations (1-5 mmol/L), chloride stimulates transport, whereas at higher concentrations (above 10 mmol/L), chloride inhibits transport. We have shown that low concentrations of chloride (1-4 mmol/L) stimulated glutamate uptake by both αTC1-9 and βTC-6, whereas concentrations higher than 10 mmol/L attenuated the stimulatory effect, which resembles that of the neuronal VGLUTs. The vesicular glutamate transport in both cells was dose dependent and saturable, with affinity (Km) of 1.5 mmol/L[8].
PHYSIOLOGICAL SIGNIFICANCE OF VESICULAR GLUTAMATE TRANSPORTERS IN DIGESTIVE SYSTEM
As glutamate release from vesicles plays an important role in regulation of insulin and glucagon exocytosis, one question of immediate interest is whether modulation of the VGLUT activity might drive changes in synaptic strength and the subsequent hormone release. Transporters responsible for vesicular uptake of classical neurotransmitters from the cytoplasm belong to three distinct families, one for monoamines and acetylcholine, another for transmitters such as GABA[48], and a third for glutamate[30,36]. Inhibition or reduced activity of the vesicular transporters can decrease the amount of transmitter stored in vesicles, because both reduced quantal size and frequency in PC12 cells[49,50] and in a model of developing neuromuscular junction[51] after pharmacological blockade of uptake transport by reserpine or vesamicol. Evidences suggested that VGLUT is directly involved in insulin secretion from pancreatic β cells, as inhibitors of VGLUT suppress the glutamate-evoked insulin exocytosis[19].
At the neuromuscular junction, convincing evidence that receptors are not saturated and that synaptic strength can be influenced by the activity of vesicular acetylcholine transporters exists[52]. Assuming glutamate receptors are not saturated by single quanta (and the balance of evidence at many synapses suggests that they are not), then increasing or decreasing the activity of a VGLUT could in principle alter synaptic strength. With these assumptions, regulated changes in the localization of VGLUT1 and VGLUT2 might also affect hormone release in pancreas as proposed for other vesicular transporters[48]. Mice that possess only one functional vesicular monoamine transporter isoform 2 (VMAT2) allele contain 50% of the dopamine and serotonin levels of wild-type animals[53-55]. Ventral midbrain cultures derived from the ventral tegmental area (VTA) of these mice show a 50% reduction in depolarization-evoked dopamine release compared to wild-type cultures, suggesting either a reduced number of vesicles capable of storing transmitter or reduced quantal size because of decreased transmitter accumulation per vesicle[53]. Therefore, modulation of VGLUT expression would have particularly important effects in the neurons and endocrine cells. It will also be interesting to study whether regulation of vesicular transporter activity occurs in response to physiological activity and what the consequences of such regulation are for excitatory signaling.
REGULATION OF VGLUTS IN DIGESTIVE SYSTEM
Considerable evidences indicate that the biosynthesis and transport of neurotransmitters undergo regulation by physiological or pathophysiological factors. For example, VMAT2 is an important vesicular transporter for histamine. Recent work has demonstrated that VMAT2 gene expression may be regulated by gastrin[56] and ovarian hormones[57]. Intracellular calcium increases vesicular monoamine transporter through a transcriptional activation mechanism in chromaffin cells[58]. Inonmycin stimulates mRNA expression for both VMAT2 in a pre-β-cell-line Ea3.123. Actinomycin D prevented this up-regulation, indicating that the increased mRNA abundance was the result of increased transcription[59].
Pancreatic glucagon and insulin secreting cells have different sensitivities to glutamate according to the glucose concentration. As glucagon and insulin are hyper- and hypoglycemic hormones respectively, it might be suggested that glutamate could play a part in the regulation of glucose homeostasis. VGLUT1 and VGLUT2 have distinct physiological regulation due to their complementary expression in CNS and pancreas. Evan blue, a competitive inhibitor of VGLUT, has been shown to increase glutamate storage capacity in NG108-15 cells via increasing of VGLUT1 mRNA expression[60]. Stimulatory glucose concentration (12.8 mmol/L) increases cellular total glutamate level in rat insulinoma INS-1 cells and this increase can be abolished by carbonyl cyanide p-trifluoromethoxyphenylhydrazone, an inhibitor of vesicular glutamate storage through VGLUT[61]. We have shown that low glucose concentration stimulates vesicular glutamate uptake in α cells, while high glucose concentration stimulates vesicular glutamate uptake in β cells[8]. The increases of VGLUT in α and β cells are due to upregulation of VGLUT2 mRNA in response to intracellular glucose levels. In contrast, expression of VGLUT1 was not changed by high glucose, although its expression was more predominant than VGLUT2 in β cell. These findings suggest that chronic exposure to low glucose concentration stimulates glutamate uptake into secretory vesicles in α cell which favors glutamate-evoked glucagon release, while chronic exposure to high glucose concentration stimulates glutamate uptake into secretory vesicles in β cell which favors glutamate-evoked insulin release. In addition, actinomycin D can suppress mRNA expressions of VGLUT2 in response to glucose indicating that transcriptional mechanisms are involved in glucose-mediated VGLUT2 regulations in both α and β cells[8].
PERSPECTIVES
Glutamate signaling in non-neuronal tissue has been discovered recently. It has been postulated that glutamate may act as a “cytokine” to influence cellular activity in peripheral tissues[1]. Glutamate signaling is involved in insulin and glucagon secretion of pancreatic islets, histamine-induced acid secretion of stomach, and contractility in the gastrointestinal tract. Differential regulation of VGLUTs by glucose in pancreatic α and β cells suggested that these transporters might play a significant role in diabetes. Further studies on regulation of these transporters in pancreatic islets might uncover new mechanisms involved in the pathogenesis of diabetes. Development of gene-specific inhibitors for VGLUT is of potential pharmaceutical interest for the treatment of diabetes and GI motility disorders.
Science Editor Guo SY Language Editor Elsevier HK