Published online Mar 21, 2005. doi: 10.3748/wjg.v11.i11.1715
Revised: April 8, 2004
Accepted: May 24, 2004
Published online: March 21, 2005
AIM: To investigate whether there was a dominant sacral root for the motive function of rectum and anal sphincter, and to provide an experimental basis for sacral root electrically stimulated defecation in spinal cord injuries.
METHODS: Eleven spinal cord injured mongrel dogs were included in the study. After L4-L7 laminectomy, the bilateral L7-S3 roots were electrostimulated separately and rectal and sphincter pressure were recorded synchronously. Four animals were implanted electrodes on bilateral S2 roots.
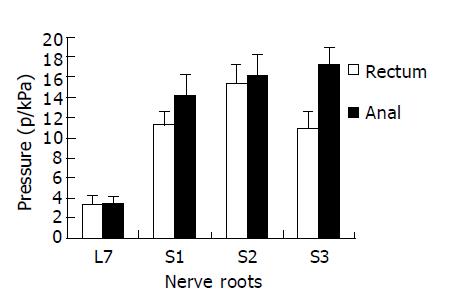
RESULTS: For rectal motorial innervation, S2 was the most dominant (mean 15.2 kPa, 37.7% of total pressure), S1 (11.3 kPa, 27.6%) and S3 (10.9 kPa, 26.7%) contributed to a smaller part. For external anal sphincter, S3 (mean 17.2 kPa, 33.7%) was the most dominant, S2 (16.2 kPa, 31.6%) and S1 (14.3 kPa, 27.9%) contributed to a lesser but still a significant part. Above 85% L7 roots provided some functional contribution to rectum and anal sphincter. For both rectum and sphincter, the right sacral roots provided more contribution than the left roots. Postoperatively, the 4 dogs had electrically stimulated defecation and micturition under the control of the neuroprosthetic device.
CONCLUSION: S2 root is the most dominant contributor to rectal pressure in dogs. Stimulation of bilateral S2 with implanted electrodes contributes to good micturition and defecation in dogs.
- Citation: Chang SM, Yu GR, Diao YM, Zhang MJ, Wang SB, Hou CL. Sacral anterior root stimulated defecation in spinal cord injuries: An experimental study in canine model. World J Gastroenterol 2005; 11(11): 1715-1718
- URL: https://www.wjgnet.com/1007-9327/full/v11/i11/1715.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i11.1715
Spinal cord injuries often occur in fairly young people, who have the prospect of an almost normal life expectancy but a considerably impaired quality of life. These patients not only experience severe dysfunction of voluntary movement of limbs but also have impaired pelvic organ functions such as bladder, bowel, and sex[1].
Urinary problems in patients with spinal cord injuries have been extensively studied, and with the advent of intermittent catheterization (CIC), electrical stimulation of the bladder, and advances in diagnostic techniques, considerable improvements have been made in strengthening the lower urinary tract and renal function. In contrast, the management of bowel disorders has remained essentially unchanged in the past three decades[2-4].
One of the most distressing aspects of spinal cord injury is not able to regulate bowel function. Patients with complete supraconal lesions lose conscious control of defecation. Although they may be able to defecate reflexly by anorectal stimulation, evacuation is often inefficient and incomplete, resulting in a high incidence of constipation[5-8].
The first Finetech-Brindley’s sacral anterior root stimulator (SARS)[9,10] was implanted in a patient with spinal cord injury in 1976. Since then, the device has been implanted in about 2000 patients suffering from complete supraconal spinal cord injury (SCI) with intact bladder innervation to induce urine evacuation[11]. The stimulator was initially developed to improve bladder emptying, but as the parasympathetic and somatic nerves that supply the distal colon, anorectum, and anal sphincter are all derived from the same sacral spinal roots that are used for electrical micturition, it seems likely that the device can also be used to induce defecation in paraplegic patients.
Several authors[12-15] have reported the clinical and manometric results of the implanted neuroprosthesis. However, experimental studies concerning electrically stimulated defecation are fen[15]. In the present study, we reported a procedure that could allow selective sacral anterior root stimulation by application of electrodes to achieve controlled rectal evacuation.
Eleven adult male mongrel dogs, weighing 12±2.5 kg (range, 10-15 kg), were used in this study. The animals were kept in cages for 1 wk prior to the study.
The dogs were with diazepam at a dose of 0.4 mg/kg body weight (bw). They were anesthetized with intravenous sodium pentobarbital at a dose of 25 mg/kg bw with a bolus injection of 15-20 mg/h to maintain adequate anesthesia with spontaneous respiration. Intravenous infusion of normal saline solution was given at a dose of 15-20 mL/kg bw per hour. All animals were administered antibiotics (penicillin) peri-operatively.
With the animal lying prone, the back was shaved and prepped with betadine solution. A supraconal spinal cord transection was made at the T10 vertebral level. Then L5-S2 laminectomy was performed. L7 and S1-3 nerve roots were exposed extradurally. They were identified anatomically and by specific motoric responses on stimulation using a bipolar hook electrode. Nerve stimulation was given using 5 to 10 trains of constant current square pulses (200 s’, 2.0 mA) delivered at a rate of 50 Hz using electromyography (Cantata-2000, Dantec, Denmark). Then the dura mater was opened in posterior midline and retracted with 3-0 silks to allow access to the conus medullaris and the cauda equina. The intradural L7-S3 roots were confirmed by their corresponding extradural roots that passed through the dura cuff[17]. By meticulous microsurgical dissection, each anterior and posterior root of L7-S3 on each side was identified and separated. Then the posterior components of sacral roots were cut and a segment of 5 mm was removed to get sacral deafferentation.
A 10F catheter was introduced into the rectum and anal canal. One balloon-ended catheter was introduced into the rectum up to 5-7 cm from the anal orifice. The balloon-catheter was filled with 50 mL of water and connected to a strain gauge pressure transducer (D3-manometrics, Medic Instrument Con., Hefei, China). Another catheter was introduced into the rectal neck (anal canal) up to 2-3 cm from the anal orifice and was also connected to another pressure transducer of the manometer. With individual root stimulated, rectal pressure and anal pressure were recorded simultaneously by the D3-manometer.
The test was performed with S2 root stimulation. By manually flow-out of the filled water, the volume of rectum balloon was diminished to 10 mL, which represented simulated stools[16].
The stimulator (Tc-2000 electrodes of bladder controller) was provided by Shanghai Tongji University[18]. The device consists of three components: the implantation part, the external control part and the testing block. The implantation part is composed of two electrodes, a connecting cable and a magnetic receiver-stimulator. The external control part is composed of a control box, a cable and a magnetic transmitter. The testing block was used to monitor whether the transmitter worked well or not.
In four animals, Tc-2000 electrodes of bladder controller were implanted and trapped bilaterally to S2 nerve roots in sacral canal. The magnetic receiver-stimulator was implanted beside the sacral incision via a subcutaneous tunnel. The implanted components were fixed by suturing to sacral periosteum and subcutaneous tissue.
After operation, these four dogs were stimulated 4 times daily by the external transmitter, with an interval of 5 burst-on and 10 burst-off, at a frequency of 36 Hz and an intensity of 12 V.
The animals survived 17-41 d (average 23 d) postoperatively. No complications were encountered during the test. Electrodes did not migrate or break. All 11 dogs were evaluate during the operation and all 4 dogs with implanted electrodes were evaluate the operation.
With differential nerve root stimulation during operation, rectum and anal pressure elevated significantly as compared with the silent rest values (mean, 3.3 kPa in this group). For rectal innervation, S2 was the most dominant (mean 15.2 kPa, 37.7% of total pressure), S1 (11.3 kPa, 27.6%) and S3 (10.9 kPa, 26.7%) contributed to a smaller part (S2>S1>S3). For sphincter, S3 (mean 17.2 kPa, 33.7%) was the most dominant, S2 (16.2 kPa, 31.6%) and S1 (14.3 kPa, 27.9%) contributed to a lesser but still a significant part (S3>S2>S1) (Figure 1). Although the efficacy of L7 root contribution to pressure elevation in rectum (3.3 kPa, 8%) and anus (3.5 kPa, 7.1%) was minimal, 85% of L7 roots (frequency) provided some function to rectum and anal sphincter. For both rectum and sphincter, the right sacral roots seemed to contribute more than the left ones.
During electrical stimulation of S2 root, the rectal neck (anal canal) pressure exceeded the rectal pressure, thus rectal balloon expulsion did not occur. However, as the pressure of anal canal reduced more rapidly than that of the rectum, rectal balloon expulsion occurred in all 11 dogs as a post-stimulus defecation, which was similar to post-stimulus voiding.
After paraplegia and electrode implantation and sacral deafferentation, the bladder of animals was electrically evacuated four times per day. During electrically stimulated micturition, defecation occurred 2-3 times per day, providing there were stools in the rectum. With electrical stimulation of S2 nerve root, defecation was successful in all 4 dogs (Figure 2). No lubrication or other bowel management was needed for the dogs during their paraplegic life (mean, 23 years) after operation.
Concomitant neuropathic bladder and bowel dysfunction are common in patients with spinal cord injury[1-8]. Bladder dysfunction in these patients is commonly attributable to hyperreflexia and detrusor-sphincter dyssynergia. However, constipation in these patients is commonly caused by fecal stasis with colonic dilation[3-7]. Constipation causes substantial morbidity, and is usually managed empirically with aids, such as laxatives, suppositories, enemas or even digital evacuation[3,4]. Indeed, fecal impaction is the most common gastrointestinal complication sustained by the patients with spinal cord injury[8]. The pathophysiology of this problem is now better understood[19,20]. Menardo et al[21], in 1987 demonstrated that the main site of stasis was in the left colon and rectum following spinal cord injury.
Anatomically, dogs have seven pairs of lumbar roots and three pairs of sacral roots. The innervations of rectal detrusor and external anal sphincter (EAS) in dogs are provided by the ventral roots of L7, S1, S2, and S3, which originated from the sacral spinal cord parasympathetic and somatic centers, respectively[17]. Our experimental study demonstrated that only a pair of the most efficacious roots (S2) was needed to produce electrically stimulated micturition and defecation.
Since large diameter fibers (somatic motor) need a smaller stimulus threshold for their excitation than small ones (parasympathetic motor), activation of the small parasympathetic fibers (detrusor) is always accompanied with the activation of the larger somatic ones (anal sphincter). Attempts to empty the rectum by sacral ventral root stimulation have always been hampered by concurrent contraction of the anal sphincter, which far exceeds that of rectum. All dogs in our study were unable to expel the rectum balloon at the time of stimulation. However, the sacral anterior root stimulator took the advantage of the differences in biomechanical characteristics of the smooth (detrusor) and striated muscles (EAS). The contraction and relaxation speed of the striated muscles was faster than those of the smooth ones. When bilateral S2 anterior roots were stimulated (the posterior component was cut as deafferentation), the rectal and anal pressures increased at the same time, and the increasing amplitude of the anal pressure was remarkably higher than that of the rectal pressure. Therefore, defecation did not occur at this time. After stimulation was stopped, the EAS relaxed instantaneously and the pressure decreased rapidly to the baseline, but the rectal detrusor relaxed slowly, this slow decay resulted in a period of rectum pressure higher than the relaxed anal pressure. Thus, a positive recto-anal pressure difference developed, and defecation might occur. The evacuation mode is called post-stimulus defecation, similar to post-stimulus voiding in urinary system[10].
For human beings, the parasympathetic outflow to left colon, rectum, and internal anal sphincter is supplied by anterior sacral roots of S2, S3, and S4. These nerve roots also innervate the external anal sphincter (EAS) via its somatic contribution. Similar sacral nerve roots also innervate bladder and its sphincter. Bladder control can be successfully achieved by using Brindley’s anterior root stimulator implant. Because most of these patients had concomitant bowel dysfunction, its effect on bowel function as defecation has been documented by Varma et al[12], in 1986, MacDonagh et al[13], in 1990, Binnie et al[14], in 1991, Chia et al[15], in 1996 and Creasey et al[11], in 2001. The mechanism of improvement in bowel function is attributed to the activation of contraction of the terminal colon and rectum, resulting in the movement of feces caudally into the anal canal. And this further initiates reflex relaxation of the pelvic floor muscles[22].
A recent intraoperative electrical study in patients with spinal cord injury demonstrated that, S3 root was the most efficacious contributor (52.2% of total pressure), S4 was the second but still significant efficacious one (44.9%), and S2 was the last and the least contributor (2.9%)[23]. The S2 root is mainly related to sexual function, and probably serves as a vital pathway for sensory feedback that is necessary for proper function of the viscera, especially for penile sensation, erection and ejaculation[24]. Based on this experimental study, the authors would like to propose a cheaper modification of Brindley’s sacral anterior root stimulated micturition and defecation in Asians, i.e., using one cable with two electrodes to trap on the bilateral S3-4 roots[25].
In conclusion, S2 root is the most dominant contributor to rectal pressure in dogs. Stimulation of bilateral S2 with implanted electrodes contributes to good micturition and defecation in dogs.
Science Editor Wang XL and Zhu LH
Language Editor Kumar M
| 1. | Longo WE, Ballantyne GH, Modlin IM. The colon, anorectum, and spinal cord patient. A review of the functional alterations of the denervated hindgut. Dis Colon Rectum. 1989;32:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Banwell JG, Creasey GH, Aggarwal AM, Mortimer JT. Management of the neurogenic bowel in patients with spinal cord injury. Urol Clin North Am. 1993;20:517-526. [PubMed] |
| 3. | Stiens SA, Bergman SB, Goetz LL. Neurogenic bowel dysfunction after spinal cord injury: clinical evaluation and rehabilitative management. Arch Phys Med Rehabil. 1997;78:S86-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 142] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Correa GI, Rotter KP. Clinical evaluation and management of neurogenic bowel after spinal cord injury. Spinal Cord. 2000;38:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Glickman S, Kamm MA. Bowel dysfunction in spinal-cord-injury patients. Lancet. 1996;347:1651-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 243] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Krogh K, Nielsen J, Djurhuus JC, Mosdal C, Sabroe S, Laurberg S. Colorectal function in patients with spinal cord lesions. Dis Colon Rectum. 1997;40:1233-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 150] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Lynch AC, Antony A, Dobbs BR, Frizelle FA. Bowel dysfunction following spinal cord injury. Spinal Cord. 2001;39:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | De Looze D, Van Laere M, De Muynck M, Beke R, Elewaut A. Constipation and other chronic gastrointestinal problems in spinal cord injury patients. Spinal Cord. 1998;36:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Brindley GS, Polkey CE, Rushton DN. Sacral anterior root stimulators for bladder control in paraplegia. Paraplegia. 1982;20:365-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 141] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Brindley GS. The first 500 patients with sacral anterior root stimulator implants: general description. Paraplegia. 1994;32:795-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 170] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Creasey GH, Grill JH, Korsten M, U HS, Betz R, Anderson R, Walter J. An implantable neuroprosthesis for restoring bladder and bowel control to patients with spinal cord injuries: a multicenter trial. Arch Phys Med Rehabil. 2001;82:1512-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Varma JS, Binnie N, Smith AN, Creasey GH, Edmond P. Differential effects of sacral anterior root stimulation on anal sphincter and colorectal motility in spinally injured man. Br J Surg. 1986;73:478-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 49] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | MacDonagh RP, Sun WM, Smallwood R, Forster D, Read NW. Control of defecation in patients with spinal injuries by stimulation of sacral anterior nerve roots. BMJ. 1990;300:1494-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 73] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Binnie NR, Smith AN, Creasey GH, Edmond P. Constipation associated with chronic spinal cord injury: the effect of pelvic parasympathetic stimulation by the Brindley stimulator. Paraplegia. 1991;29:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Chia YW, Lee TK, Kour NW, Tung KH, Tan ES. Microchip implants on the anterior sacral roots in patients with spinal trauma: does it improve bowel function? Dis Colon Rectum. 1996;39:690-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Shafik A. Sacral root stimulation for controlled defecation. Eur Surg Res. 1995;27:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Hassouna M, Li JS, Elhilali M. Dog as an animal model for neurostimulation. Neurourol Urodyn. 1994;13:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Diao YM, Lei B, Zhang MJ, Wang SB. Electrical stimulation mechanism of detrusor-sphincter coordination and its clinical application. Tongji Daxue Xuebao. 2002;30:1402-1405. |
| 19. | Gonella J, Bouvier M, Blanquet F. Extrinsic nervous control of motility of small and large intestines and related sphincters. Physiol Rev. 1987;67:902-961. [PubMed] |
| 20. | Glick ME, Meshkinpour H, Haldeman S, Hoehler F, Downey N, Bradley WE. Colonic dysfunction in patients with thoracic spinal cord injury. Gastroenterology. 1984;86:287-294. [PubMed] |
| 21. | Menardo G, Bausano G, Corazziari E, Fazio A, Marangi A, Genta V, Marenco G. Large-bowel transit in paraplegic patients. Dis Colon Rectum. 1987;30:924-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 73] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Sun WM, MacDonagh R, Forster D, Thomas DG, Smallwood R, Read NW. Anorectal function in patients with complete spinal transection before and after sacral posterior rhizotomy. Gastroenterology. 1995;108:990-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Chang SM, Hou CL. The frequency and efficacy of differential sacral roots innervation to bladder detrusor in Asian people. Spinal Cord. 2000;38:773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Jezernik S, Craggs M, Grill WM, Creasey G, Rijkhoff NJ. Electrical stimulation for the treatment of bladder dysfunction: current status and future possibilities. Neurol Res. 2002;24:413-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Chang SM, Hou CL, Xu RS, Diao YM, Fu XH, Wang SB, Chen AM. Sacral anterior root stimulated micturition in Chinese spinal cord injury patients: simplification and modification. Jiefangjun Yixue Zazhi. 2003;28:670-672. |