Published online Mar 14, 2005. doi: 10.3748/wjg.v11.i10.1549
Revised: September 3, 2004
Accepted: September 15, 2004
Published online: March 14, 2005
AIM: The cag pathogenicity island (PAI) is one of potential virulence determinants of Helicobacter pylori. The Mongolian gerbil is a suitable experimental animal for the screening of virulence factors of H pylori.
METHODS: Five-week-old Mongolian gerbils were inoculated with a standard H pylori strain (ATCC 43504) possessing the cag PAI or a clinical isolate lacking the genes’ cluster (OHPC-0002). The animals were killed at 2, 4, 8, 24 and 48 wk after inoculation (n = 5 each), and macroscopic and histopathological findings in the stomachs were compared.
RESULTS: In gerbils infected with ATCC 43504, a more severe degree of infiltration of polynuclear and mononuclear cells and lymphoid follicles was observed from 4 wk after inoculation compared to gerbils infected with OHPC-0002 especially in the antrum and transitional zone from the fundic to pyloric gland area. In addition, glandular atrophy, intestinal metaplasia, gastric ulcer and hyperplastic polyps were noted in gerbils infected with ATCC 43504, whereas only mild gastric erosions occurred in those infected with OHPC-0002.
CONCLUSION: Our results indicate that the cag PAI could be directly involved in gastric immune and inflammatory responses in the Mongolian gerbils, leading to a more advanced gastric disease.
-
Citation: Ohnita K, Isomoto H, Honda S, Wada A, Wen CY, Nishi Y, Mizuta Y, Hirayama T, Kohno S.
Helicobacter pylori strain-specific modulation of gastric inflammation in Mongolian gerbils. World J Gastroenterol 2005; 11(10): 1549-1553 - URL: https://www.wjgnet.com/1007-9327/full/v11/i10/1549.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i10.1549
Helicobacter pylori (H pylori) is a Gram-negative, spiral-shaped, microaerophilic bacterium that colonizes the human gastric mucosa[1-3]. The prevalence of H pylori infection is still high in Asian countries including Japan, and this organism is now recognized as a major cause of chronic active gastritis[1-4], peptic ulcers[2-6] and gastric malignancies[4,5,7,8]. However, the clinical manifestation of H pylori infection varies widely in infected individuals and most persons are asymptomatic all their life[9]. The pathogenic mechanism of this pleomorphism is not completely understood, but might be explained, in part, by strain diversity[10-13].
Several factors have been proposed as possible virulence determinants[14-18]. Among them, the cag pathogenicity island (cag PAI), a 40-kb cluster of -30 genes of supposedly extraneous origin[19], is reportedly associated with advanced gastroduodenal diseases including gastric cancer[11-14].
In an effort to develop a model that would parallel as close as possible the course of infection as it occurs in humans, Hirayama et al[20] established an experimental model of inbred Mongolian gerbils with persistent H pylori infection. Infection of Mongolian gerbils with H pylori results in chronic active gastritis, gastric ulcer, intestinal metaplasia and gastric cancer[21-24]. The Mongolian gerbil model may be valuable not only in elucidating H pylori-related neoplasia but also in evaluating virulence factors[11,12]. We sought to investigate the chronological and spatial changes of gastric inflammation after inoculation of H pylori strains possessing or lacking the cag PAI.
Five-week-old male specific pathogen-free Mongolian gerbils (MGS/Sea, male) weighting 27-43 g (Seac Yoshitomi, Fukuoka, Japan) were used. They were housed four per cage in stainless-steel cages on hardwood chip bedding in an air-conditioned room (12/12 h light/dark cycle) at 24 °C. They were fasted for 24 h prior to H pylori inoculation, and then fed with chow (CE-2; Clea Japan Co., Tokyo, Japan) and distilled water ad libitum 12 h after inoculation. Experiments were performed according to the guidelines of Ethics Committee for Animal Experiments at Nagasaki University.
We used the ATCC 43504 standard strain of H pylori known to possess cag PAI and the cag PAI-totally deleted strain OHPC-0002[25]. The H pylori strains were cultivated on Muller Hinton II agar plate with 7% horse blood at 37 °C for 3 d under microaerophilic conditions[25]. Bacteria harvested from the plates were incubated in 200 mL of Brucella broth (DIFCO Laboratories, Detroit, MI) with 10% horse serum at 37 °C for 24 h with vigorous shaking under controlled microaerophilic atmosphere[23,25]. The inoculum size was adjusted with sterile saline to produce the optical density of McFarland 4 at 540 nm[23].
A total of 75 Mongolian gerbils were randomly assigned to three experimental groups (25 each group). The animals were challenged with the vehicle (group A), 109 colony-forming units (CFU) ATCC 43504 (group B) or OHPC-0002 (group C) in 1 mL of Brucella broth with 10% horse serum by intragastric gavage. Five gerbils in each experimental group were sacrificed under anesthesia at 2, 4, 8, 24 and 48 wk after inoculation. The stomachs were quickly removed, opened along the greater curvature, divided into two, and used for histopathological and culture examinations.
Half the stomach was fixed in 10% neutral buffered formalin and embedded in paraffin. Four µm thick sections were stained with hematoxylin and eosin. According to the Sydney system, each histological parameter of activity (neutrophils), chronic inflammation (mononuclear cells), glandular atrophy and intestinal metaplasia was graded into none, mild, moderate or severe[26]. Intestinal metaplasia was defined by the presence of goblet cells in glandular mucosa with Alcian blue (pH 2.5)/periodic acid-Schiff staining[27]. The biopsy specimens were examined blindly without knowledge of the results of the experimental group.
The remaining half of the stomach was minced in 2 mL of Brucella broth, placed on the blood agar and incubated for 4 d at 37 °C under the microaerophilic conditions[23].
H pylori was recovered from almost all gerbils of groups B and C from 4 wk after inoculation throughout the whole observation period. The bacterial densities assessed by Giemsa staining did not differ significantly between groups B and C.
There were no visible changes in the stomach of Mongolian gerbils of group A throughout the whole observation period. Until 4 wk after inoculation, no gloss appearance was noted in the stomach of both groups B and C. At 8 wk, erosions with bleeding appeared in the antrum and transitional zone extending from fundic to pyloric mucosa in all the animals of group B. In one gerbil of the group B, the stomach contained an ulcer in the vicinity of the transitional zone at 8 wk post-inoculation. The erosive lesions were noted in the transitional mucosa of 3 and 4 animals of group B at 24 and 48 wk respectively. Sessile polyps with occasional apical erosions, which were classified histopathologically as hyperplastic polyps, were seen in the antrum of one Mongolian gerbil of group B. On the other hand, there were no visible macroscopic findings in the stomach of Mongolian gerbils of group C until 24 wk after inoculation. Mild erosions were seen in the antrum of one gerbil and in the transitional zone of 2 gerbils of group C at 48 wk.
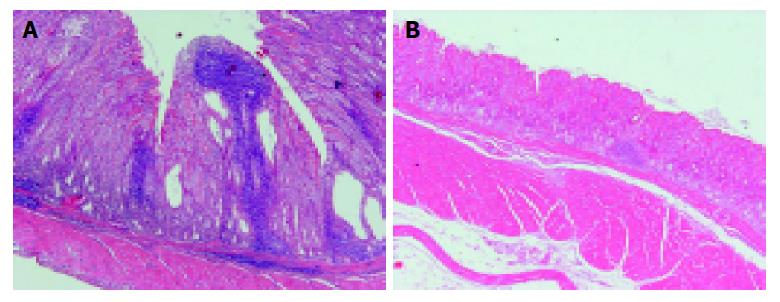
No histopathological changes were noted in the stomach of Mongolian gerbils of group A throughout the whole observation period. Table 1 compares the histopathological grades of gastritis noted in the mucosa of gerbils of groups B and C based on the Sydney classification. At 2 wk after inoculation, mild mucosal infiltration of polynuclear and mononuclear cells appeared in the antrum of one gerbil of group B. At 4 wk, moderate to severe chronic active gastritis consisting of mononuclear and polynuclear cells was noted in the lamina propria of the antrum and transitional zone of group B. The histopathological changes in group B reached peak levels between 8 to 24 wk (Figure 1A). Lymphoid follicles were especially conspicuous in the submucosa but they were also found in the deep portion of the mucosa of almost all animals of group B from 4 wk after inoculation. In the fundic mucosa of group B, mild mononuclear and polynuclear cell infiltration appeared at 4 wk. Mild to moderate infiltration of neutrophils and mononuclear cells was noted in the corpus of some gerbils of group B up to 48 wk, but the grades of gastritis were less severe than those in the antrum and the transitional zone. Mild atrophic gastritis appeared in the antrum and transitional zone of Mongolian gerbils of group B at 4 wk after inoculation, but seen in the corpus of only one gerbil at 24 wk. Intestinal metaplasia of incomplete type was found in the antrum of one gerbil of group B.
| Post-inoculation interval (wk) | |||||||||||||||
| Antrum | Transitional zone | Corpus | |||||||||||||
| 2 | 4 | 8 | 24 | 48 | 2 | 4 | 8 | 24 | 48 | 2 | 4 | 8 | 24 | 48 | |
| ATCC 43504-infected mongolian gerbils | |||||||||||||||
| Activity | |||||||||||||||
| None | 4 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 0 | 1 | 5 | 3 | 2 | 3 | 4 |
| Mild | 1 | 2 | 2 | 2 | 4 | 0 | 2 | 0 | 1 | 2 | 0 | 2 | 2 | 2 | 0 |
| Moderate | 0 | 3 | 2 | 3 | 0 | 0 | 2 | 2 | 4 | 2 | 0 | 0 | 1 | 0 | 1 |
| Severe | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chronic inflammation | |||||||||||||||
| None | 4 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 5 | 2 | 0 | 2 | 2 |
| Mild | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 3 | 3 | 1 | 3 |
| Moderate | 0 | 2 | 1 | 2 | 2 | 0 | 3 | 3 | 1 | 2 | 0 | 0 | 2 | 2 | 0 |
| Severe | 0 | 3 | 4 | 3 | 3 | 0 | 0 | 2 | 4 | 2 | 0 | 0 | 0 | 0 | 0 |
| Glandular atrophy | |||||||||||||||
| None | 5 | 2 | 2 | 2 | 2 | 5 | 4 | 2 | 2 | 1 | 5 | 5 | 5 | 4 | 5 |
| Mild | 0 | 3 | 3 | 3 | 3 | 0 | 1 | 3 | 3 | 4 | 0 | 0 | 0 | 1 | 0 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Intestinal metaplasia | |||||||||||||||
| None | 5 | 5 | 5 | 5 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Mild | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ohpc-0002-infected mongolian gerbils | |||||||||||||||
| Activity | |||||||||||||||
| None | 5 | 5 | 4 | 5 | 3 | 5 | 5 | 5 | 5 | 3 | 5 | 5 | 5 | 5 | 4 |
| Mild | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chronic inflammation | |||||||||||||||
| None | 5 | 5 | 4 | 2 | 1 | 5 | 5 | 5 | 4 | 3 | 5 | 5 | 5 | 5 | 4 |
| Mild | 0 | 0 | 1 | 3 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Moderate | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Glandular atrophy | |||||||||||||||
| None | 5 | 5 | 4 | 3 | 4 | 5 | 5 | 5 | 4 | 3 | 5 | 5 | 5 | 4 | 5 |
| Mild | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 0 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Intestinal metaplasia | |||||||||||||||
| None | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Mild | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Moderate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
On the other hand, some Mongolian gerbils of group C showed mild mononuclear and polynuclear cell infiltration in the antral mucosa at 8 wk after inoculation (Figure 1B). Furthermore, mild to moderate chronic active gastritis was noted in the antrum and transitional zone at 48 wk in some animals. Glandular atrophy appeared in the antral and transitional mucosa at 8 wk but was exclusively mild. No intestinal metaplasia was present anywhere in the animals of group C.
Various animals have been used for studying experimental H pylori infection including monkeys, dogs, piglets, domestic cats, rats and nude mice[28-31]. These animal models had disadvantages such as low infection rates, instability, immunodeficiency and high costs[11,32]. Infection of conventional mice with H pylori strain SS1 overcame many of the above problems, but gastritis in mice induced by this infection is less intense than in humans[33]. In 1996, Hirayama et al[20] reported the development of a successful Mongolian gerbil model with persistent H pylori infection. Several investigators confirmed successively the progression from chronic active gastritis to atrophic gastritis and then to intestinal metaplasia and the occurrence of gastric ulcer in the Mongolian gerbil model[22,23]. In addition, long-term H pylori infection by itself resulted even in the development of gastric adenocarcinoma[24]. Thus, the gerbil seems to be the best experimental model for H pylori infection, and we employed the Mongolian gerbil to unravel the impact of cag PAI status, an important virulence determinant, on gastric pathogenesis in vivo.
In Mongolian gerbils infected with ATCC 43504 with total cag PAI, mild infiltration of neutrophils and mononuclear cells appeared in the antrum at 2 wk after inoculation. From 4 wk, moderate to severe chronic active gastritis, often accompanied by lymphoid follicle formation, was noted predominantly in the antrum and transitional zone. In the fundic mucosa, the severity of gastritis worsened to moderate from 8 wk. Such chronological changes of histological gastritis and spatial shift from antral to corporeal mucosa observed in gerbils infected with ATCC 43504 were consistent with previous results in Mongolian gerbils inoculated with other standard or clinical strains possessing intact cag PAI[11,12,22,23].
The major finding of the present study is that the strain possessing the cag PAI induced more severe degree of gastritis in Mongolian gerbils than clinical strain lacking the genes’ cluster[25]. Because there were no differences in the susceptibility and bacterial density between the two strains, ATCC 43504 and OHPC-0002, the deletion did not change the ability of bacterial colonization or proliferation but affected the inflammatory reactions in the stomach of Mongolian gerbils. Akanuma et al[12] cited similar results; a genetically handled mutant lacking total cag PAI or knockout strain of cagE, one of the functional genes in cag PAI, ameliorated histopathological gastritis in this animal. In addition, glandular atrophy, incomplete intestinal metaplasia, gastric ulcer and hyperplastic polyps were noted in gerbils infected with the cag PAI-positive strains, whereas only mild gastric erosions concomitant with slight infiltration of inflammatory cells occurred in animals infected with the cag PAI-negative strains. These findings indicate that intact cag PAI could be directly involved in the host immune and inflammatory processes and cause more advanced gastric diseases.
Deletions of the cag PAI and several cag insertion mutations reportedly block the induction of proinflammatory cytokine, interleukin-8, in gastric epithelial cell lines[19,34]. The expression of interleukin-8 gene is up-regulated by nuclear factor kappa B (NF-κB), which is involved in various inflammatory conditions such as inflammatory bowel diseases, rheumatoid arthritis and H pylori-associated gastritis through the regulation of a plethora of genes that encode bioactive molecules that mediate various immune and inflammatory responses[35-37]. In fact, accumulating evidence indicates that intact cag PAI is a prerequisite for the activation of NF-κB[34]. Considered together, these results suggest that the cag PAI-mediated activation of NF-κB plays an important role in the pathogenesis of gastritis in Mongolian gerbils.
The VacA protein, which causes vacuolation in cultured cells[17,18], is responsible for the virulence of H pylori and ulcer formation in the stomach and duodenum[10,38]. However, since the VacA-positive strains often have intact cag PAI, the involvement of VacA in inducing gastritis is still controversial[11,39]. In this regard, the VacA-disrupted H pylori strain could cause inflammatory changes in the stomach of Mongolian gerbils to a degree similar to those seen in Mongolian gerbils infected with the wild strain[11], indicating that VacA is not crucial for gastric inflammation. Several other candidate genes outside the cag PAI[15,16] should be validated in this reliable rodent model for the screening of determinants of virulence of H pylori.
Edited by Li WZ Language Editor Elsevier HK
| 1. | Blaser MJ. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990;161:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 478] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 2. | Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 398] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | Götz JM, Veenendaal RA, Biemond I, Muller ES, Veselic M, Lamers CB. Serum gastrin and mucosal somatostatin in Helicobacter pylori-associated gastritis. Scand J Gastroenterol. 1995;30:1064-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2805] [Cited by in RCA: 2739] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 5. | Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1302] [Cited by in RCA: 1234] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 6. | Graham DY, Lew GM, Klein PD, Evans DG, Evans DJ, Saeed ZA, Malaty HM. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. A randomized, controlled study. Ann Intern Med. 1992;116:705-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 622] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 7. | Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, Isaacson PG. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1564] [Cited by in RCA: 1386] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 8. | Morris A, Nicholson G. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am J Gastroenterol. 1987;82:192-199. [PubMed] |
| 9. | Dooley CP, Cohen H, Fitzgibbons PL, Bauer M, Appleman MD, Perez-Perez GI, Blaser MJ. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989;321:1562-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 499] [Article Influence: 13.9] [Reference Citation Analysis (2)] |
| 10. | Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 621] [Article Influence: 29.6] [Reference Citation Analysis (1)] |
| 11. | Ogura K, Maeda S, Nakao M, Watanabe T, Tada M, Kyutoku T, Yoshida H, Shiratori Y, Omata M. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J Exp Med. 2000;192:1601-1610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 231] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Akanuma M, Maeda S, Ogura K, Mitsuno Y, Hirata Y, Ikenoue T, Otsuka M, Watanabe T, Yamaji Y, Yoshida H. The evaluation of putative virulence factors of Helicobacter pylori for gastroduodenal disease by use of a short-term Mongolian gerbil infection model. J Infect Dis. 2002;185:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Azuma T, Ohtani M, Yamazaki Y, Higashi H, Hatakeyama M. Meta-analysis of the relationship between CagA seropositivity and gastric cancer. Gastroenterology. 2004;126:1926-1927; author reply 1927-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Azuma T, Yamazaki S, Yamakawa A, Ohtani M, Muramatsu A, Suto H, Ito Y, Dojo M, Yamazaki Y, Kuriyama M. Association between diversity in the Src homology 2 domain--containing tyrosine phosphatase binding site of Helicobacter pylori CagA protein and gastric atrophy and cancer. J Infect Dis. 2004;189:820-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Dorrell N, Martino MC, Stabler RA, Ward SJ, Zhang ZW, McColm AA, Farthing MJ, Wren BW. Characterization of Helicobacter pylori PldA, a phospholipase with a role in colonization of the gastric mucosa. Gastroenterology. 1999;117:1098-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Yamaoka Y, Kwon DH, Graham DY. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc Natl Acad Sci USA. 2000;97:7533-7538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 318] [Article Influence: 12.7] [Reference Citation Analysis (2)] |
| 17. | Padilla PI, Wada A, Yahiro K, Kimura M, Niidome T, Aoyagi H, Kumatori A, Anami M, Hayashi T, Fujisawa J. Morphologic differentiation of HL-60 cells is associated with appearance of RPTPbeta and induction of Helicobacter pylori VacA sensitivity. J Biol Chem. 2000;275:15200-15206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Wada A, Yahiro K, Hirayama T. Helicobacter pylori vacuolating cytotoxin (VacA) and its modulatory effects in host cells. Tanpakushitsu Kakusan Koso. 2001;46:519-523. [PubMed] |
| 19. | Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648-14653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1375] [Cited by in RCA: 1392] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 20. | Hirayama F, Takagi S, Kusuhara H, Iwao E, Yokoyama Y, Ikeda Y. Induction of gastric ulcer and intestinal metaplasia in mongolian gerbils infected with Helicobacter pylori. J Gastroenterol. 1996;31:755-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Fujioka T, Honda S, Tokieda M. Helicobacter pylori infection and gastric carcinoma in animal models. J Gastroenterol Hepatol. 2000;15 Suppl:D55-D59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Ikeno T, Ota H, Sugiyama A, Ishida K, Katsuyama T, Genta RM, Kawasaki S. Helicobacter pylori-induced chronic active gastritis, intestinal metaplasia, and gastric ulcer in Mongolian gerbils. Am J Pathol. 1999;154:951-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 118] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Honda S, Fujioka T, Tokieda M, Gotoh T, Nishizono A, Nasu M. Gastric ulcer, atrophic gastritis, and intestinal metaplasia caused by Helicobacter pylori infection in Mongolian gerbils. Scand J Gastroenterol. 1998;33:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Honda S, Fujioka T, Tokieda M, Satoh R, Nishizono A, Nasu M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255-4259. [PubMed] |
| 25. | Wada A, Mori N, Oishi K, Hojo H, Nakahara Y, Hamanaka Y, Nagashima M, Sekine I, Ogushi K, Niidome T. Induction of human beta-defensin-2 mRNA expression by Helicobacter pylori in human gastric cell line MKN45 cells on cag pathogenicity island. Biochem Biophys Res Commun. 1999;263:770-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Price AB. The Sydney System: histological division. J Gastroenterol Hepatol. 1991;6:209-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 655] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 27. | Isomoto H, Mizuta Y, Inoue K, Matsuo T, Hayakawa T, Miyazaki M, Onita K, Takeshima F, Murase K, Shimokawa I. A close relationship between Helicobacter pylori infection and gastric xanthoma. Scand J Gastroenterol. 1999;34:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Karita M, Li Q, Cantero D, Okita K. Establishment of a small animal model for human Helicobacter pylori infection using germ-free mouse. Am J Gastroenterol. 1994;89:208-213. [PubMed] |
| 29. | Lambert JR, Borromeo M, Pinkard KJ, Turner H, Chapman CB, Smith ML. Colonization of gnotobiotic piglets with Campylobacter Pyloridis--an animal model? J Infect Dis. 1987;155:1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Radin MJ, Eaton KA, Krakowka S, Morgan DR, Lee A, Otto G, Fox J. Helicobacter pylori gastric infection in gnotobiotic beagle dogs. Infect Immun. 1990;58:2606-2612. [PubMed] |
| 31. | Shuto R, Fujioka T, Kubota T, Nasu M. Experimental gastritis induced by Helicobacter pylori in Japanese monkeys. Infect Immun. 1993;61:933-939. [PubMed] |
| 32. | Yan J, Luo YH, Mao YF. Establishment of Helicobacter pylori infection model in Mongolian gerbils. World J Gastroenterol. 2004;10:852-855. [PubMed] |
| 33. | Lee A, O'Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 758] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 34. | Ogura K, Takahashi M, Maeda S, Ikenoue T, Kanai F, Yoshida H, Shiratori Y, Mori K, Mafune KI, Omata M. Interleukin-8 production in primary cultures of human gastric epithelial cells induced by Helicobacter pylori. Dig Dis Sci. 1998;43:2738-2743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Hirata Y, Maeda S, Mitsuno Y, Tateishi K, Yanai A, Akanuma M, Yoshida H, Kawabe T, Shiratori Y, Omata M. Helicobacter pylori CagA protein activates serum response element-driven transcription independently of tyrosine phosphorylation. Gastroenterology. 2002;123:1962-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2488] [Cited by in RCA: 2492] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 37. | Isomoto H, Mizuta Y, Miyazaki M, Takeshima F, Omagari K, Murase K, Nishiyama T, Inoue K, Murata I, Kohno S. Implication of NF-kappaB in Helicobacter pylori-associated gastritis. Am J Gastroenterol. 2000;95:2768-2776. [PubMed] |
| 38. | Fujikawa A, Shirasaka D, Yamamoto S, Ota H, Yahiro K, Fukada M, Shintani T, Wada A, Aoyama N, Hirayama T. Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nat Genet. 2003;33:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 39. | Maeda S, Ogura K, Yoshida H, Kanai F, Ikenoue T, Kato N, Shiratori Y, Omata M. Major virulence factors, VacA and CagA, are commonly positive in Helicobacter pylori isolates in Japan. Gut. 1998;42:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 183] [Article Influence: 6.8] [Reference Citation Analysis (0)] |